Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90
- PMID: 8962087
- PMCID: PMC26168
- DOI: 10.1073/pnas.93.25.14536
Pharmacologic shifting of a balance between protein refolding and degradation mediated by Hsp90
Abstract
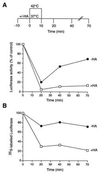
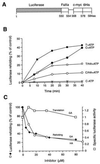
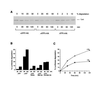
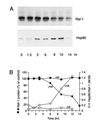
The role of the abundant stress protein Hsp90 in protecting cells against stress-induced damage is not well understood. The recent discovery that a class of ansamycin antibiotics bind specifically to Hsp90 allowed us to address this problem from a new angle. We find that mammalian Hsp90, in cooperation with Hsp70, p60, and other factors, mediates the ATP-dependent refolding of heat-denatured proteins, such as firefly luciferase. Failure to refold results in proteolysis. The ansamycins inhibit refolding, both in vivo and in a cell extract, by preventing normal dissociation of Hsp90 from luciferase, causing its enhanced degradation. This mechanism also explains the ansamycin-induced proteolysis of several protooncogenic protein kinases, such as Raf-1, which interact with Hsp90. We propose that Hsp90 is part of a quality control system that facilitates protein refolding or degradation during recovery from stress. This function is used by a limited set of signal transduction molecules for their folding and regulation under nonstress conditions. The ansamycins shift the mode of Hsp90 from refolding to degradation, and this effect is probably amplified for specific Hsp90 substrates.
Figures


References
-
- Craig E A, Gross C A. Trends Biochem Sci. 1991;16:135–140. - PubMed
-
- Parsell D A, Lindquist S. Annu Rev Genet. 1993;27:437–496. - PubMed
-
- Georgopoulos C, Liberek K, Zylicz M, Ang D. Properties of the Heatshock Proteins of Escherichia coli and the Autoregulation of the Heat Shock Response. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. pp. 209–249.
-
- Wiech H, Buchner J, Zimmermann R, Jacob U. Nature (London) 1992;358:169–170. - PubMed
-
- Yonehara M, Minami Y, Kawata Y, Nagai J, Yahara I. J Biol Chem. 1996;271:2641–2645. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

