Transfected Drosophila cells as a probe for defining the minimal requirements for stimulating unprimed CD8+ T cells
- PMID: 8962124
- PMCID: PMC26205
- DOI: 10.1073/pnas.93.25.14736
Transfected Drosophila cells as a probe for defining the minimal requirements for stimulating unprimed CD8+ T cells
Abstract
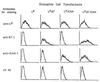
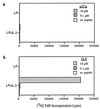
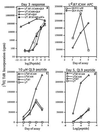
Stimulation of naive T cells by antigen-presenting cells (APC) is thought to involve two qualitatively different signals: signal one results from T-cell receptor (TCR) recognition of antigenic peptides bound to major histocompatibility complex (MHC) molecules, whereas signal two reflects contact with one or more costimulatory molecules. The requirements for stimulating naive T cells were studied with MHC class I-restricted CD8+ T cells from a T-cell receptor transgenic line, with defined peptides as antigen and transfected Drosophila cells as APC. Three main findings are reported. First, stimulation of naive T cells via signal one alone (MHC plus peptide) was essentially nonimmunogenic; thus T cells cultured with peptides presented by MHC class I-transfected Drosophila APC lacking costimulatory molecules showed little or no change in their surface phenotype. Second, cotransfection of two costimulatory molecules, B7-1 and intercellular adhesion molecule 1 (ICAM-1), converted class I+ Drosophila cells to potent APC capable of inducing strong T-proliferative responses and cytokine (interleukin 2) production. Third, B7-1 and ICAM-1 acted synergistically, indicating that signal two is complex; synergy between B7-1 and ICAM-1 varied from moderate to extreme and was influenced by both the dose and affinity of the peptide used and the parameter of T-cell activation studied. Transfected Drosophila cells are thus a useful tool for examining the minimal APC requirements for naive T cells.
Figures

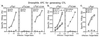
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

