Melanoma angiogenesis and metastasis modulated by ribozyme targeting of the secreted growth factor pleiotrophin
- PMID: 8962127
- PMCID: PMC26208
- DOI: 10.1073/pnas.93.25.14753
Melanoma angiogenesis and metastasis modulated by ribozyme targeting of the secreted growth factor pleiotrophin
Abstract
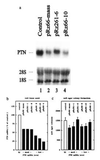
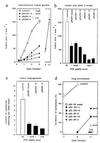
Clinical and experimental evidence suggests that spreading of malignant cells from a localized tumor (metastasis) is directly related to the number of microvessels in the primary tumor. This tumor angiogenesis is thought to be mediated by tumor-cell-derived growth factors. However, most tumor cells express a multitude of candidate angiogenesis factors and it is difficult to decipher which of these are rate-limiting factors in vivo. Herein we use ribozyme targeting of pleiotrophin (PTN) in metastatic human melanoma cells to assess the significance of this secreted growth factor for angiogenesis and metastasis. As a model we used human melanoma cells (1205LU) that express high levels of PTN and metastasize from subcutaneous tumors to the lungs of experimental animals. In these melanoma cells, we reduced PTN mRNA and growth factor activity by transfection with PTN-targeted ribozymes and generated cell lines expressing different levels of PTN. We found that the reduction of PTN does not affect growth of the melanoma cells in vitro. In nude mice, however, tumor growth and angiogenesis were decreased in parallel with the reduced PTN levels and apoptosis in the tumors was increased. Concomitantly, the metastatic spread of the tumors from the subcutaneous site to the lungs was prevented. These studies support a direct link between tumor angiogenesis and metastasis through a secreted growth factor and identify PTN as a candidate factor that may be rate-limiting for human melanoma metastasis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

