The TEL/platelet-derived growth factor beta receptor (PDGF beta R) fusion in chronic myelomonocytic leukemia is a transforming protein that self-associates and activates PDGF beta R kinase-dependent signaling pathways
- PMID: 8962143
- PMCID: PMC26224
- DOI: 10.1073/pnas.93.25.14845
The TEL/platelet-derived growth factor beta receptor (PDGF beta R) fusion in chronic myelomonocytic leukemia is a transforming protein that self-associates and activates PDGF beta R kinase-dependent signaling pathways
Abstract
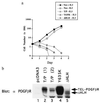
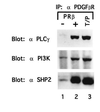
The TEL/PDGF beta R fusion protein is the product of the t(5;12) translocation in patients with chronic myelomonocytic leukemia. The TEL/PDGF beta R is an unusual fusion of a putative transcription factor, TEL, to a receptor tyrosine kinase. The translocation fuses the amino terminus of TEL, containing the helix-loop-helix (HLH) domain, to the transmembrane and cytoplasmic domain of the PDGF beta R. We hypothesized that TEL/PDGF beta R self-association, mediated by the HLH domain of TEL, would lead to constitutive activation of the PDGF beta R tyrosine kinase domain and cellular transformation. Analysis of in vitro-translated TEL/ PDGF beta R confirmed that the protein self-associated and that self-association was abrogated by deletion of 51 aa within the TEL HLH domain. In vivo, TEL/PDGF beta R was detected as a 100-kDa protein that was constitutively phosphorylated on tyrosine and transformed the murine hematopoietic cell line Ba/F3 to interleukin 3 growth factor independence. Transformation of Ba/F3 cells required the HLH domain of TEL and the kinase activity of the PDGF beta R portion of the fusion protein. Immunoblotting demonstrated that TEL/PDGF beta R associated with multiple signaling molecules known to associate with the activated PDGF beta R, including phospholipase C gamma 1, SHP2, and phosphoinositol-3-kinase. TEL/PDGF beta R is a novel transforming protein that self-associates and activates PDGF beta R-dependent signaling pathways. Oligomerization of TEL/PDGF beta R that is dependent on the TEL HLH domain provides further evidence that the HLH domain, highly conserved among ETS family members, is a self-association motif.
Figures

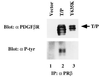

References
-
- Claesson-Welsh L. J Biol Chem. 1994;269:32023–32026. - PubMed
-
- Shurtleff S, Buijs A, Behm F, Rubnitz J, Raimondi S, Hancock M, Chan G, Pui C, Grosveld G, Downing J. Leukemia. 1995;9:1985–1989. - PubMed
-
- Papadopoulos P, Ridge S A, Boucher C A, Stocking C, Wiedemann L M. Cancer Res. 1995;55:34–38. - PubMed
-
- Buijs A, Sherr S, van Baal S, van Bezouw S, van der Plas D, van Kessel A D, Riegman P, Deprez R L, Zwarthoff E, Hagemeijer A, Grosveld G. Oncogene. 1995;10:1511–1519. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

