Resting memory CD8+ T cells are hyperreactive to antigenic challenge in vitro
- PMID: 8976170
- PMCID: PMC2196370
- DOI: 10.1084/jem.184.6.2141
Resting memory CD8+ T cells are hyperreactive to antigenic challenge in vitro
Abstract
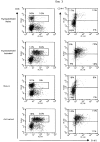
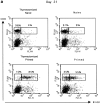
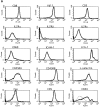
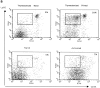
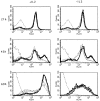
The characteristics of CD8+ T cells responsible for memory responses are still largely unknown. Particularly, it has not been determined whether different activation thresholds distinguish naive from memory CD8+ T cell populations. In most experimental systems, heterogeneous populations of primed CD8+ T cells can be identified in vivo after immunization. These cells differ in terms of cell cycle status, surface phenotype, and/or effector function. This heterogeneity has made it difficult to assess the activation threshold and the relative role of these subpopulations in memory responses. In this study we have used F5 T cell receptor transgenic mice to generate a homogeneous population of primed CD8+ T cells. In the F5 transgenic mice, peptide injection in vivo leads to activation of most peripheral CD8+ T cells. In vivo BrdU labeling has been used to follow primed T cells over time periods spanning several weeks after peptide immunization. Our results show that the majority of primed CD8+ T cells generated in this system are not cycling and express increased levels of CD44 and Ly6C. These cells remain responsive to secondary peptide challenge in vivo as evidenced by short term upregulation of activation markers such as CD69 and CD44. The activation thresholds of naive and primed CD8+ T cells were compared in vitro. We found that CD8+ T cells from primed mice are activated by peptide concentrations 10-50-fold lower than naive mice. In addition, the kinetics of interleukin 2R alpha chain upregulation by primed CD8+ T cells differ from naive CD8+ T cells. These primed hyperresponsive CD8+ T cells might play an important role in the memory response.
Figures






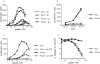
References
-
- Ahmed R, Gray D. Immunological memory and protective immunity: understanding their relation. Science (Wash DC) 1996;272:54–60. - PubMed
-
- McHeyzer-Williams MG, Davis MM. Antigenspecific development of primary and memory T cells in vivo. Science (Wash DC) 1995;268:106–111. - PubMed
-
- Horgan KJ, Van Seventer GA, Shimizu Y, Shaw S. Hyporesponsiveness of naive (CD45RA+) human T cells to multiple receptor-mediated stimuli but augmentation of responses by co-stimuli. Eur J Immunol. 1990;20:1111–1118. - PubMed
-
- Sanders ME, Makgoba MW, June CH, Young HA, Shaw S. Enhanced responsiveness of human memory T cells to CD2 and CD3 receptor-mediated activation. Eur J Immunol. 1989;19:803–808. - PubMed
-
- Kearney ER, Pape KA, Loh DY, Jenkins MK. Visualization of peptide-specific T cell immunity and peripheral tolerance induction in vivo. Immunity. 1994;1:327–339. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

