The two membrane isoforms of human IgE assemble into functionally distinct B cell antigen receptors
- PMID: 8976175
- PMCID: PMC2196392
- DOI: 10.1084/jem.184.6.2197
The two membrane isoforms of human IgE assemble into functionally distinct B cell antigen receptors
Abstract
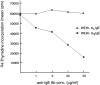
The human C epsilon gene expresses two membrane IgE heavy chain mRNAs which differ in the sequence that encodes their extracellular membrane-proximal domain. In the long IgE isoform (mLIgE), this domain contains a stretch of 52 amino acids which are absent in the short variant (mSIgE). We have now generated B cell transfectoma cell lines that express these two isoforms and show that both types of mIgE form functional B cell antigen receptors (BCR). Both receptors associate with the Ig-alpha/Ig-beta heterodimer, as well as with protein kinases that are capable of phosphorylating this complex. Upon their cross-linking, both receptors can activate protein tyrosine kinases that phosphorylate the same substrate proteins. Both IgE receptors also associate with two novel proteins that do not bind to mIgM. Apart from these similarities, the two IgE-BCRs show several differences of which some are analogous to the differences between the IgM- and IgD-BCRs. First, the mSIgE is transported to the cell surface at a higher rate than the mLIgE. Second, the two IgE-BCRs associate with differently glycosylated Ig-alpha proteins, the mLIgE associates with the completely glycosylated form, whereas the mSIgE associates with an Ig-alpha glycoform that is partially sensitive to endoglycosidase H. Third, the kinetics of protein tyrosine phosphorylation induced by receptor cross-linking is significantly different for the two IgE-BCRs. Finally, cross-linking of the mSIgE-BCR leads to growth inhibition of the B cell transfectoma, whereas signaling through the mLIgE-BCR does not affect the cellular proliferation. These data show that the two human membrane IgE isoforms assemble into functionally distinct antigen receptors which can induce different cellular responses.
Figures


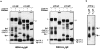


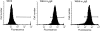
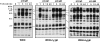
Similar articles
-
The extracellular membrane-proximal domain of membrane-bound IgE restricts B cell activation by limiting B cell antigen receptor surface expression.Eur J Immunol. 2018 Mar;48(3):441-453. doi: 10.1002/eji.201747196. Epub 2017 Dec 1. Eur J Immunol. 2018. PMID: 29150831
-
Targeting the junction of CɛmX and ɛ-migis for the specific depletion of mIgE-expressing B cells.Mol Immunol. 2012 Oct;52(3-4):279-88. doi: 10.1016/j.molimm.2012.06.004. Epub 2012 Jun 29. Mol Immunol. 2012. PMID: 22750228
-
Aberrant trafficking of the B cell receptor Ig-alpha beta subunit in a B lymphoma cell line.J Immunol. 2000 Aug 1;165(3):1427-37. doi: 10.4049/jimmunol.165.3.1427. J Immunol. 2000. PMID: 10903747
-
Signal transduction by the B-cell antigen receptor.Ann N Y Acad Sci. 1995 Sep 7;766:195-201. doi: 10.1111/j.1749-6632.1995.tb26662.x. Ann N Y Acad Sci. 1995. PMID: 7486656 Review.
-
Isotype Specific Assembly of B Cell Antigen Receptors and Synergism With Chemokine Receptor CXCR4.Front Immunol. 2018 Dec 18;9:2988. doi: 10.3389/fimmu.2018.02988. eCollection 2018. Front Immunol. 2018. PMID: 30619343 Free PMC article. Review.
Cited by
-
IgE-expressing long-lived plasma cells in persistent sensitization.Front Pediatr. 2022 Dec 5;10:979012. doi: 10.3389/fped.2022.979012. eCollection 2022. Front Pediatr. 2022. PMID: 36545659 Free PMC article. Review.
-
Signalling of the BCR is regulated by a lipid rafts-localised transcription factor, Bright.EMBO J. 2009 Mar 18;28(6):711-24. doi: 10.1038/emboj.2009.20. Epub 2009 Feb 12. EMBO J. 2009. PMID: 19214191 Free PMC article.
-
Anti-IgE therapy for IgE-mediated allergic diseases: from neutralizing IgE antibodies to eliminating IgE+ B cells.Clin Transl Allergy. 2018 Jul 18;8:27. doi: 10.1186/s13601-018-0213-z. eCollection 2018. Clin Transl Allergy. 2018. PMID: 30026908 Free PMC article. Review.
-
Past, present, and future of anti-IgE biologics.Allergy. 2020 Oct;75(10):2491-2502. doi: 10.1111/all.14308. Epub 2020 Apr 21. Allergy. 2020. PMID: 32249957 Free PMC article. Review.
-
B Cell Intrinsic Mechanisms Constraining IgE Memory.Front Immunol. 2017 Nov 13;8:1277. doi: 10.3389/fimmu.2017.01277. eCollection 2017. Front Immunol. 2017. PMID: 29180995 Free PMC article. Review.
References
-
- DeFranco AL. Structure and function of the B cell antigen receptor. Annu Rev Cell Biol. 1993;9:377–410. - PubMed
-
- Hombach J, Tsubata T, Leclercq L, Stappert H, Reth M. Molecular components of the B-cell antigen receptor complex of the IgM class. Nature (Lond) 1990;43:760–762. - PubMed
-
- Reth M. Antigen receptors on B lymphocytes. Annu Rev Immunol. 1992;10:97–121. - PubMed
-
- Reth M. B cell antigen receptors. Curr Opin Immunol. 1994;6:3–8. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

