The route of antigen entry determines the requirement for L-selectin during immune responses
- PMID: 8976188
- PMCID: PMC2196391
- DOI: 10.1084/jem.184.6.2341
The route of antigen entry determines the requirement for L-selectin during immune responses
Abstract
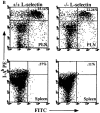
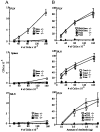
L-selectin, an adhesion molecule constitutively expressed on leukocytes, is important for primary adhesion and extravasation of lymphocytes at specialized high endothelial venules within lymph nodes and other leukocytes at sites of inflammation. We have generated L-selectin-deficient mice by targeted disruption, and have confirmed a previously reported phenotype which includes strikingly impaired contact hypersensitivity (CHS) responses to reactive haptens (Tedder, T.F., D.A. Steeber, and P. Pizcueta. 1995. J. Exp. Med. 181:2259-2264; Xu, J.C., I.S. Grewal, G.P. Geba, and R.A. Flavell. 1996. 183:589-598.). Since the mechanism of this impairment has not been clarified, we sought to define the stage(s) at which the CHS response is affected in L-selectin-deficient mice. We show that epidermal Langerhans cells in L-selectin-deficient mice are normal in number, migrate to peripheral lymph nodes appropriately, and are functional in presenting allogeneic and haptenic antigens. Moreover, T cells, as well as neutrophil and monocyte effector populations, are fully capable of entry into the inflamed skin sites in the absence of L-selectin. Thus, antigen presentation and effector mechanisms are intact in L-selectin deficient mice. In contrast, virtually no antigen-specific T cells can be found within draining peripheral nodes after a contact challenge, suggesting that the defect resides primarily in the inability of antigen-specific T cells to home to and be activated in these nodes. Indeed, L-selectin-deficient mice mount completely normal CHS responses when alternate routes of immunization are used. These studies pinpoint the lesion in CHS to a discrete stage of the afferent limb of the response, clarify the role of L-selectin on effector populations, and illustrate the critical importance of the route of antigen entry to the successful execution of an immune response.
Figures








References
-
- Gowans JL, Knight EJ. The route of recirculation of lymphocytes in the rat. Philos Trans R Soc Lond Ser B Biol Sci. 1964;159:257–282. - PubMed
-
- Gallatin WM, Weissman IL, Butcher EC. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature (Lond) 1983;304:30–34. - PubMed
-
- Carlos TM, Harlan JM. Leukocyte-endothelial adhesion molecules. Blood. 1994;84:2068–2101. - PubMed
-
- Lawrence MB, Springer TA. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991;65:859–873. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

