Multiple sclerosis: Fas signaling in oligodendrocyte cell death
- PMID: 8976190
- PMCID: PMC2196365
- DOI: 10.1084/jem.184.6.2361
Multiple sclerosis: Fas signaling in oligodendrocyte cell death
Abstract
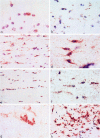
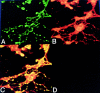
Fas is a cell surface receptor that transduces cell death signals when cross-linked by agonist antibodies or by fas ligand. In this study, we examined the potential of fas to contribute to oligodendrocyte (OL) injury and demyelination as they occur in the human demyelinating disease multiple sclerosis (MS). Immunohistochemical study of central nervous system (CNS) tissue from MS subjects demonstrated elevated fas expression on OLs in chronic active and chronic silent MS lesions compared with OLs in control tissue from subjects with or without other neurologic diseases. In such lesions, microglia and infiltrating lymphocytes displayed intense immunoreactivity to fas ligand. In dissociated glial cell cultures prepared from human adult CNS tissue, fas expression was restricted to OLs. Fas ligation with the anti-fas monoclonal antibody M3 or with the fas-ligand induced rapid OL cell membrane lysis, assessed by LDH release and trypan blue uptake and subsequent cell death. In contrast to the activity of fas in other cellular systems, dying OLs did not exhibit evidence of apoptosis, assessed morphologically and by terminal transferase-mediated d-uridine triphosphate-biotin nick-end-labeling staining for DNA fragmentation. Other stimuli such as C2-ceramide were capable of inducing rapid apoptosis in OLs. Antibodies directed at other surface molecules expressed on OLs or the M33 non-activating anti-fas monoclonal antibody did not induce cytolysis of OLs. Our results suggest that fas-mediated signaling might contribute in a novel cytolytic manner to immune-mediated OL injury in MS.
Figures



Similar articles
-
Fas expression on human fetal astrocytes without susceptibility to fas-mediated cytotoxicity.Neuroscience. 1998 May;84(2):627-34. doi: 10.1016/s0306-4522(97)00455-7. Neuroscience. 1998. PMID: 9539231
-
Oligodendrocyte injury in multiple sclerosis: a role for p53.J Neurochem. 2003 May;85(3):635-44. doi: 10.1046/j.1471-4159.2003.01674.x. J Neurochem. 2003. PMID: 12694389
-
Oligodendrogliopathy in Multiple Sclerosis: Low Glycolytic Metabolic Rate Promotes Oligodendrocyte Survival.J Neurosci. 2016 Apr 27;36(17):4698-707. doi: 10.1523/JNEUROSCI.4077-15.2016. J Neurosci. 2016. PMID: 27122029 Free PMC article.
-
Multiple sclerosis and central nervous system demyelination.J Autoimmun. 1999 Nov;13(3):297-306. doi: 10.1006/jaut.1999.0321. J Autoimmun. 1999. PMID: 10550217 Review.
-
Mechanisms of tissue injury in multiple sclerosis: opportunities for neuroprotective therapy.J Neural Transm Suppl. 2000;(58):193-203. doi: 10.1007/978-3-7091-6284-2_16. J Neural Transm Suppl. 2000. PMID: 11128609 Review.
Cited by
-
Gelatinase B/matrix metalloproteinase-9 is a phase-specific effector molecule, independent from Fas, in experimental autoimmune encephalomyelitis.PLoS One. 2018 Oct 1;13(10):e0197944. doi: 10.1371/journal.pone.0197944. eCollection 2018. PLoS One. 2018. PMID: 30273366 Free PMC article.
-
Dendritic cells overexpressing Fas-ligand induce pulmonary vasculitis in mice.Clin Exp Immunol. 2004 Jul;137(1):74-80. doi: 10.1111/j.1365-2249.2004.02514.x. Clin Exp Immunol. 2004. PMID: 15196246 Free PMC article.
-
T-cell apoptosis in inflammatory brain lesions: destruction of T cells does not depend on antigen recognition.Am J Pathol. 1998 Sep;153(3):715-24. doi: 10.1016/s0002-9440(10)65615-5. Am J Pathol. 1998. PMID: 9736022 Free PMC article.
-
Perforin-dependent neurologic injury in a viral model of multiple sclerosis.J Neurosci. 1998 Sep 15;18(18):7306-14. doi: 10.1523/JNEUROSCI.18-18-07306.1998. J Neurosci. 1998. PMID: 9736651 Free PMC article.
-
Fas/Apo [apoptosis]-1 and associated proteins in the differentiating cerebral cortex: induction of caspase-dependent cell death and activation of NF-kappaB.J Neurosci. 1999 Mar 1;19(5):1754-70. doi: 10.1523/JNEUROSCI.19-05-01754.1999. J Neurosci. 1999. PMID: 10024361 Free PMC article.
References
-
- Dawson JW. The histology of disseminated sclerosis. Trans R Soc Edin. 1916;50:517–540.
-
- Lumsden, C.E. 1955. Neuropathology of multiple sclerosis. In Multiple Sclerosis. D. McAlpine, N.D. Compston, and C.E. Lumsden, editors. Livingstone Press Inc., Edinburgh. 208–293.
-
- Prineas, J.W. 1985. Neuropathology of multiple sclerosis. In Handbook of Clinical Neurology: Demyelinating Diseases. P.J. Vinken, G.W. Bruyn, and H.L. Klawans, editors. Elsevier Science Publishing Co. Inc., New York. 213–257.
-
- Raine, C.S. 1990. Demyelinating diseases. In Textbook of Neuropathology. R.L. Davis and D.M. Robertson, editors. Williams and Wilkins, Baltimore, MD. 535–620.
-
- Raine CS. The Dale E. McFarlin Memorial Lecture: the immunology of the multiple sclerosis lesion. Ann Neurol. 1994;36:561–572. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

