Viral infection of transgenic mice expressing a viral protein in oligodendrocytes leads to chronic central nervous system autoimmune disease
- PMID: 8976191
- PMCID: PMC2196376
- DOI: 10.1084/jem.184.6.2371
Viral infection of transgenic mice expressing a viral protein in oligodendrocytes leads to chronic central nervous system autoimmune disease
Abstract
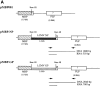
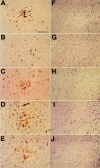
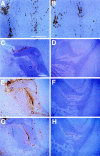
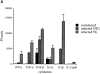
One hypothesis for the etiology of central nervous system (CNS) autoimmune disease is that infection by a virus sharing antigenic epitopes with CNS antigens (molecular mimicry) elicits a virus-specific immune response that also recognizes self-epitopes. To address this hypothesis, transgenic mice were generated that express the nucleoprotein or glycoprotein of lymphocytic choriomeningitis virus (LCMV) as self in oligodendrocytes. Intraperitoneal infection with LCMV strain Armstrong led to infection of tissues in the periphery but not the CNS, and the virus was cleared within 7-14 d. After clearance, a chronic inflammation of the CNS resulted, accompanied by upregulation of CNS expression of MHC class I and II molecules. A second LCMV infection led to enhanced CNS pathology, characterized by loss of myelin and clinical motor dysfunction. Disease enhancement also occurred after a second infection with unrelated viruses that cross-activated LCMV-specific memory T cells. These findings indicate that chronic CNS autoimmune disease may be induced by infection with a virus sharing epitopes with a protein expressed in oligodendrocytes and this disease may be enhanced by a second infection with the same or an unrelated virus. These results may explain the association of several different viruses with some human autoimmune diseases.
Figures





Similar articles
-
Using transgenic mouse models to dissect the pathogenesis of virus-induced autoimmune disorders of the islets of Langerhans and the central nervous system.Immunol Rev. 1996 Aug;152:111-43. doi: 10.1111/j.1600-065x.1996.tb00913.x. Immunol Rev. 1996. PMID: 8930670 Review.
-
In vivo treatment with a MHC class I-restricted blocking peptide can prevent virus-induced autoimmune diabetes.J Immunol. 1998 Nov 1;161(9):5087-96. J Immunol. 1998. PMID: 9794447
-
Virus-specific MHC-class II-restricted TCR-transgenic mice: effects on humoral and cellular immune responses after viral infection.Eur J Immunol. 1998 Jan;28(1):390-400. doi: 10.1002/(SICI)1521-4141(199801)28:01<390::AID-IMMU390>3.0.CO;2-O. Eur J Immunol. 1998. PMID: 9485218
-
Pathological changes in the islet milieu precede infiltration of islets and destruction of beta-cells by autoreactive lymphocytes in a transgenic model of virus-induced IDDM.J Autoimmun. 1997 Jun;10(3):231-8. doi: 10.1006/jaut.1997.0131. J Autoimmun. 1997. PMID: 9218748
-
T and B cell tolerance and responses to viral antigens in transgenic mice: implications for the pathogenesis of autoimmune versus immunopathological disease.Immunol Rev. 1991 Aug;122:133-71. doi: 10.1111/j.1600-065x.1991.tb00601.x. Immunol Rev. 1991. PMID: 1937540 Review.
Cited by
-
Viruses and autoimmunity: an affair but not a marriage contract.Rev Med Virol. 2002 Mar-Apr;12(2):107-13. doi: 10.1002/rmv.347. Rev Med Virol. 2002. PMID: 11921306 Free PMC article. Review.
-
Molecular mimicry: its evolution from concept to mechanism as a cause of autoimmune diseases.Monoclon Antib Immunodiagn Immunother. 2014 Jun;33(3):158-65. doi: 10.1089/mab.2013.0090. Epub 2014 Apr 2. Monoclon Antib Immunodiagn Immunother. 2014. PMID: 24694269 Free PMC article.
-
Virus et auto-immunité.Ann Inst Pasteur Actual. 1996;7(2):81-86. doi: 10.1016/S0924-4204(97)85202-X. Epub 2000 Mar 5. Ann Inst Pasteur Actual. 1996. PMID: 32288234 Free PMC article. French.
-
Antiviral immune responses: triggers of or triggered by autoimmunity?Nat Rev Immunol. 2009 Apr;9(4):246-58. doi: 10.1038/nri2527. Nat Rev Immunol. 2009. PMID: 19319143 Free PMC article. Review.
-
Molecular mimicry and multiple sclerosis: degenerate T-cell recognition and the induction of autoimmunity.Ann Neurol. 1999 May;45(5):559-67. doi: 10.1002/1531-8249(199905)45:5<559::aid-ana3>3.0.co;2-q. Ann Neurol. 1999. PMID: 10319877 Free PMC article. Review.
References
-
- Jahnke U, Fischer EH, Alvord ECJ. Sequence homology between certain viral proteins and proteins related to encephalomyelitis and neuritis. Science (Wash DC) 1985;229:282–284. - PubMed
-
- Fujinami RS, Oldstone MBA. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: a mechanism for autoimmunity. Science (Wash DC) 1985;230:1043–1045. - PubMed
-
- Fujinami RS. Virus-induced autoimmunity through molecular mimicry. Ann NY Acad Sci. 1988;540:210–217. - PubMed
-
- Oldstone MBA. Molecular mimicry and autoimmune disease. Cell. 1987;50:819–820. - PubMed
-
- Hall R. Molecular mimicry. Adv Parasitol. 1994;34:81–132. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

