Immunoglobulin G-mediated inflammatory responses develop normally in complement-deficient mice
- PMID: 8976192
- PMCID: PMC2196366
- DOI: 10.1084/jem.184.6.2385
Immunoglobulin G-mediated inflammatory responses develop normally in complement-deficient mice
Abstract
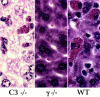
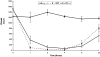
The role of complement in immunoglobulin G-triggered inflammation was studied in mice genetically deficient in complement components C3 and C4. Using the reverse passive Arthus reaction and experimental models of immune hemolytic anemia and immune thrombocytopenia, we show that these mice have types II and III inflammatory responses that are indistinguishable from those of wild-type animals. Complement-deficient and wild-type animals exhibit comparable levels of erythrophagocytosis and platelet clearance in response to cytotoxic anti-red blood cell and antiplatelet antibodies. Furthermore, in the reverse passive Arthus reaction, soluble immune complexes induce equivalent levels of hemmorhage, edema, and neutrophillic infiltration in complement-deficient and wild-type animals. In contrast, mice that are genetically deficient in the expression of Fc receptors exhibit grossly diminished reactions by both cytotoxic antibodies and soluble immune complexes. These studies provide strong evidence that the activation of cell-based Fc gamma R receptors, but not complement, are required for antibody-triggered murine inflammatory responses.
Figures




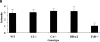
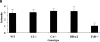

References
-
- Roitt, I., J. Brostoff, and D. Male. 1993. In Immunology, 3rd ed. Mosby, Inc., London. 20.1–20.2.
-
- Snyder, E.L. 1995. Transfusion reactions. In Hematology, Basic Principles and Practice, 2nd ed. R. Hoffman, E.J. Benz, S.J. Shattol, B. Furie, H. Cohen, and L.E. Silberstein, editors. Churchill Livingstone Inc., New York.) 2045–2053.
-
- Gallin, J.I. 1993. Inflammation. In Fundamental Immunology, 3rd ed., W. Paul, editor. Raven Press, New York. 1015–1032.
-
- Arthus M. Injections repetees de serum de cheval cuez le lapin. C R Soc Biol. 1903;55:817–820.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

