Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis
- PMID: 8986765
- PMCID: PMC26357
- DOI: 10.1073/pnas.93.26.15069
Peroxynitrite, the coupling product of nitric oxide and superoxide, activates prostaglandin biosynthesis
Abstract
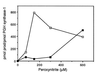
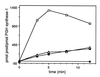
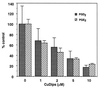
Peroxynitrite activates the cyclooxygenase activities of constitutive and inducible prostaglandin endoperoxide synthases by serving as a substrate for the enzymes' peroxidase activities. Activation of purified enzyme is induced by direct addition of peroxynitrite or by in situ generation of peroxynitrite from NO coupling to superoxide anion. Cu,Zn-superoxide dismutase completely inhibits cyclooxygenase activation in systems where peroxynitrite is generated in situ from superoxide. In the murine macrophage cell line RAW264.7, the lipophilic superoxide dismutase-mimetic agents, Cu(II) (3,5-diisopropylsalicylic acid)2, and Mn(III) tetrakis(1-methyl-4-pyridyl)porphyrin dose-dependently decrease the synthesis of prostaglandins without affecting the levels of NO synthase or prostaglandin endoperoxide synthase or by inhibiting the release of arachidonic acid. These findings support the hypothesis that peroxynitrite is an important modulator of cyclooxygenase activity in inflammatory cells and establish that superoxide anion serves as a biochemical link between NO and prostaglandin biosynthesis.
Figures


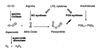
References
-
- Needleman P, Turk J, Jakschik B A, Morrison A R, Lefkowith J B. Annu Rev Biochem. 1986;55:69–102. - PubMed
-
- Smith W L, Marnett L J. In: Metal Ions in Biological Systems. Sigel H, Sigel A, editors. Vol. 30. Basel: Dekker; 1994. pp. 163–199.
-
- Nugteren D H, Hazelhof E. Biochim Biophys Acta. 1973;326:448–461. - PubMed
-
- Vane J, Botting R. FASEB J. 1988;2:89–96. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

