Architectural limits on split genes
- PMID: 8986767
- PMCID: PMC26359
- DOI: 10.1073/pnas.93.26.15081
Architectural limits on split genes
Abstract
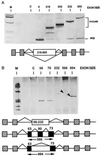
Exon/intron architecture varies across the eukaryotic kingdom with large introns and small exons the rule in vertebrates and the opposite in lower eukaryotes. To investigate the relationship between exon and intron size in pre-mRNA processing, internally expanded exons were placed in vertebrate genes with small and large introns. Both exon and intron size influenced splicing phenotype. Intron size dictated if large exons were efficiently recognized. When introns were large, large exons were skipped; when introns were small, the same large exons were included. Thus, large exons were incompatible for splicing if and only if they were flanked by large introns. Both intron and exon size became problematic at approximately 500 nt, although both exon and intron sequence influenced the size at which exons and introns failed to be recognized. These results indicate that present-day gene architecture reflects at least in part limitations on exon recognition. Furthermore, these results strengthen models that invoke pairing of splice sites during recognition of pre-mRNAs, and suggest that vertebrate consensus sequences support pairing across either introns or exons.
Figures


References
-
- Moore M J, Query C C, Sharp P A. In: RNA World. Gesteland R F, Atkins J F, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. pp. 305–358.
-
- Ruby S, Abelson J. Trends Genet. 1991;7:79–85. - PubMed
-
- Guthrie C. Science. 1991;253:157–163. - PubMed
-
- Berget S M. J Biol Chem. 1995;270:2411–2414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

