Direct interaction of flagellin termini essential for polymorphic ability of flagellar filament
- PMID: 8986772
- PMCID: PMC26364
- DOI: 10.1073/pnas.93.26.15108
Direct interaction of flagellin termini essential for polymorphic ability of flagellar filament
Abstract
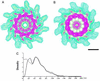
We report the structures of flagellar filaments reconstituted from various flagellins with small terminal truncations. Flagellins from Salmonella typhimurium strains SJW1103 (wild type), SJW1660, and SJW1655 were used, which form a left-handed supercoil, the L- and R-type straight forms, respectively. Structure analyses were done by electron cryomicroscopy and helical image reconstruction with a help of x-ray fiber diffraction for determining precise helical symmetries. Truncation of either terminal region, irrespective of the original flagellin species, results in a straight filament having a helical symmetry distinct either from the L- or R-type. This filament structure is named Lt-type. Although the local subunit packing is similar in all three types, a close comparison shows that the Lt-type packing is almost identical to the R-type but distinct from the L-type, which demonstrates the strong two-state preference of the subunit interactions. The structure clearly suggests that both termini are located in the inner tube of the concentric double-tubular structure of the filament core, and their proper interaction is responsible for the correct folding of fairly large terminal regions that form the inner tube. The double tubular structure appears to be essential for the polymorphic ability of flagellar filaments, which is required for the swimming-tumbling of bacterial taxis.
Figures


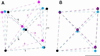
References
-
- Macnab R M, Ornston M K. J Mol Biol. 1977;112:1–30. - PubMed
-
- Larsen S H, Reader R W, Kort E N, Tso W W, Adler J. Nature (London) 1974;249:74–77. - PubMed
-
- Berg H C, Anderson R A. Nature (London) 1973;245:380–382. - PubMed
-
- Silverman M, Simon M. Nature (London) 1974;249:73–74. - PubMed
-
- Asakura S. Adv Biophys. 1970;1:99–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

