DNA damage and p53-mediated cell cycle arrest: a reevaluation
- PMID: 8986789
- PMCID: PMC26382
- DOI: 10.1073/pnas.93.26.15209
DNA damage and p53-mediated cell cycle arrest: a reevaluation
Abstract
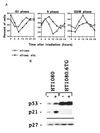
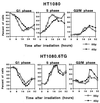
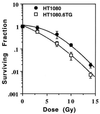
Most mammalian cells exhibit transient delays in the G1 and G2 phases of the cell cycle after treatment with radiation or radiomimetic compounds. p53 is required for the arrest in G1, which provides time for DNA repair. Recently, a role of p53 in the G2/M transition has also been suggested. However, it has been reported that the presence of functional p53 does not always correlate with the induction of these checkpoints. To precisely assess the role of p53 in activating cell cycle checkpoints and in cell survival after radiation, we studied the response of two isogenic human fibrosarcoma cell lines differing in their p53 status (wild type or mutant). We found that when irradiated cells undergo a wild-type p53-dependent G1 arrest, they do not subsequently arrest in G2. Moreover, wild-type p53 cells irradiated past the G1 checkpoint arrest in G2 but do not delay in the subsequent G1 phase. Furthermore, in these cell lines, which do not undergo radiation-induced apoptosis, the wild-type p53 cell line exhibited a greater radioresistance in terms of clonogenic survival. These results suggest that the two checkpoints may be interrelated, perhaps through a control system that determines, depending on the extent of the damage, whether the cell needs to arrest cell cycle progression at the subsequent checkpoint for further repair. p53 could be a crucial component of this control system.
Figures
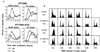
References
-
- Hartwell L H, Weinert T A. Science. 1989;246:629–634. - PubMed
-
- Hartwell L H, Kastan M B. Science. 1994;266:1821–1828. - PubMed
-
- Kastan M B, Onyekwere O, Sidransky D, Vogelstein B, Craig R. Cancer Res. 1991;51:6304–6311. - PubMed
-
- Clarke A R, Purdie C A, Harrison D J, Morris R G, Bird C C, Hooper M L, Wyllie A H. Nature (London) 1993;362:849–852. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

