Induction of HSP70 and polyubiquitin expression associated with plant virus replication
- PMID: 8986804
- PMCID: PMC26397
- DOI: 10.1073/pnas.93.26.15289
Induction of HSP70 and polyubiquitin expression associated with plant virus replication
Abstract
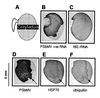
By examining the front of virus invasion in immature pea embryos infected with pea seed-borne mosaic virus (PSbMV), the selective control of different host genes has been observed. From our observations, the early responses to PSbMV replication can be grouped into three classes, inhibited host gene expression, induced host gene expression, and no effect on a normal host function. The expression of two heat-inducible genes encoding HSP70 and polyubiquitin was induced coordinately with the onset of virus replication and the down-regulation of two other genes encoding lipoxygenase and heat shock cognate protein. The down-regulation was part of a general suppression of host gene expression that may be achieved through the degradation of host transcripts. We discuss the possibilities of whether the induction of HSP70 and polyubiquitin genes represents a requirement for the respective protein products by the virus or is merely a consequence of the depletion of other host transcripts. The former is feasible, as the induction of both genes does result in increased HSP70 and ubiquitin accumulation. This also indicates that, in contrast to some animal virus infections, there is not a general inhibition of translation of host mRNAs following PSbMV infection. This selective control of host gene expression was observed in all cell types of the embryo and identifies mechanisms of cellular disruption that could act as triggers for symptom expression.
Figures



References
-
- Knipe D M. In: Fundamental Virology. 3rd Ed. Fields B N, Knipe D M, Howley P M, editors. Philadelphia: Lippincott–Raven; 1996. pp. 239–265.
-
- Wang D, Maule A J. Science. 1995;267:229–231. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

