Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach
- PMID: 8986818
- PMCID: PMC26411
- DOI: 10.1073/pnas.93.26.15370
Cell surface expression of the epithelial Na channel and a mutant causing Liddle syndrome: a quantitative approach
Abstract
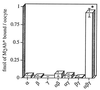
The epithelial amiloride-sensitive sodium channel (ENaC) controls transepithelial Na+ movement in Na(+)-transporting epithelia and is associated with Liddle syndrome, an autosomal dominant form of salt-sensitive hypertension. Detailed analysis of ENaC channel properties and the functional consequences of mutations causing Liddle syndrome has been, so far, limited by lack of a method allowing specific and quantitative detection of cell-surface-expressed ENaC. We have developed a quantitative assay based on the binding of 125I-labeled M2 anti-FLAG monoclonal antibody (M2Ab*) directed against a FLAG reporter epitope introduced in the extracellular loop of each of the alpha, beta, and gamma ENaC subunits. Insertion of the FLAG epitope into ENaC sequences did not change its functional and pharmacological properties. The binding specificity and affinity (Kd = 3 nM) allowed us to correlate in individual Xenopus oocytes the macroscopic amiloride-sensitive sodium current (INa) with the number of ENaC wild-type and mutant subunits expressed at the cell surface. These experiments demonstrate that: (i) only heteromultimeric channels made of alpha, beta, and gamma ENaC subunits are maximally and efficiently expressed at the cell surface; (ii) the overall ENaC open probability is one order of magnitude lower than previously observed in single-channel recordings; (iii) the mutation causing Liddle syndrome (beta R564stop) enhances channel activity by two mechanisms, i.e., by increasing ENaC cell surface expression and by changing channel open probability. This quantitative approach provides new insights on the molecular mechanisms underlying one form of salt-sensitive hypertension.
Figures




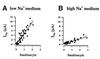
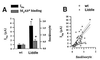
References
-
- Canessa C M, Schild L, Buell G, Thorens B, Gautschi I, Horisberger J-D, Rossier B C. Nature (London) 1994;367:463–467. - PubMed
-
- Lingueglia E, Renard S, Voilley N, Waldmann R, Chassande O, Lazdunski M, Barbry P. Eur J Biochem. 1993;216:679–687. - PubMed
-
- Mcdonald F J, Price M P, Snyder P M, Welsh M J. Am J Physiol. 1995;268:C1157–C1163. - PubMed
-
- Puoti A, May A, Canessa C M, Horisberger J D, Schild L, Rossier B C. Am J Physiol. 1995;38:C188–C197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

