L-selectin activates the Ras pathway via the tyrosine kinase p56lck
- PMID: 8986819
- PMCID: PMC26412
- DOI: 10.1073/pnas.93.26.15376
L-selectin activates the Ras pathway via the tyrosine kinase p56lck
Abstract
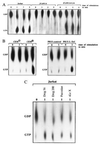
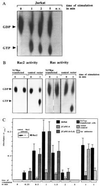
Selectins mediate rolling, the initial step of leukocyte adhesion to endothelial cells [Springer, T. A. (1995) Annu. Rev. Physiol. 57, 827-872 and Butcher, E. C. (1991) Cell 67, 1033-1036]. In this study we show that L-selectin triggering of Jurkat cells using different antibodies or glycomimetics resulted in activation of the src-tyrosine kinase p56lck; tyrosine phosphorylation of intracellular proteins, in particular mitogen-activating protein kinase and L-selectin; and association of Grb2/Sos with L-selectin. This association correlated with an activation of p21Ras, mitogen-activating protein kinase, Rac2, and a transient increase of 2-O synthesis. Stimulation of the Ras pathway by L-selectin requires functional p56lck, since p56lck-deficient Jurkat cells (JCaM1.6) do not show tyrosine phosphorylation, association of L-selectin with Grb2/Sos, and activation of Ras upon L-selectin triggering. Transfection of JCaM1.6 cells with p56lck reconstitutes the observed signaling events. Genetic inhibition of Ras or Rac2 prevented Rac2 stimulation and 2-O synthesis, respectively. The specificity and the physiological significance of the observed signaling cascade is indicated by stimulation of L-selectin-transfected P815, L-selectin-positive CEM or peripheral blood lymphocytes resulting in the same activation events as in Jurkat cells. Our results point to a signaling cascade from L-selectin via p56lck, Grb2/Sos, Ras, and Rac2 to 2-O.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

