A novel subunit for shal K+ channels radically alters activation and inactivation
- PMID: 8987734
- PMCID: PMC6793676
- DOI: 10.1523/JNEUROSCI.17-01-00032.1997
A novel subunit for shal K+ channels radically alters activation and inactivation
Abstract
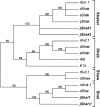
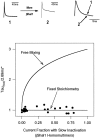
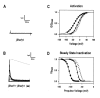
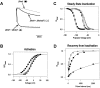
Shal (Kv4) potassium channel genes encode classical subthreshold A-currents, and their regulation may be a key factor in determining neuronal firing frequency. The inactivation rate of Shal channels is increased by a presently unidentified class of proteins in both Drosophila and mammals. We have cloned a novel Shal channel subunit (jShalgamma1) from the jellyfish Polyorchis penicillatus that alters Shal currents from both invertebrates and vertebrates. When co-expressed with the conserved jellyfish Shal homolog jShal1, jShalgamma1 dramatically changes both the rate of inactivation and voltage range of activation and steady-state inactivation. jShalgamma1 provides fast inactivation by a classic N-type mechanism, which is independent of its effects on voltage dependence. jShalgamma1 forms functional channels only as a heteromultimer, and jShalgamma1 + jShal1 heteromultimers are functional only in a 2:2 subunit stoichiometry.
Figures



References
-
- Anderson PAV. Ionic basis of action potentials and bursting activity in the hydromedusan jellyfish Polyorchis penicillatus. J Exp Biol. 1979;78:299–302.
-
- Anderson PAV, McKay MC. The electrophysiology of cnidocytes. J Exp Biol. 1987;133:215–230.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
