Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition
- PMID: 8987736
- PMCID: PMC6793687
- DOI: 10.1523/JNEUROSCI.17-01-00058.1997
Single-channel properties of recombinant AMPA receptors depend on RNA editing, splice variation, and subunit composition
Abstract
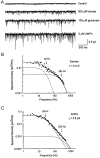
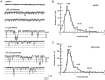
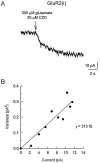
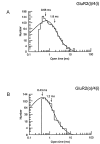
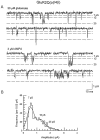
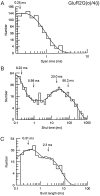
Non-NMDA glutamate receptor subunits of the AMPA-preferring subfamily combine to form ion channels with heterogeneous functional properties. We have investigated the effects of RNA editing at the Q/R site, splice variation of the "flip/flop" cassette, and multimeric subunit assembly on the single-channel conductance and kinetic properties of the recombinant AMPA receptors formed from GluR2 and GluR4 expressed in HEK 293 cells. We found that AMPA receptor single-channel conductance was dependent on the Q/R site editing state of the subunits comprising the channel. Calcium-permeable (unedited) channels had resolvable single-channel events with main conductance states of 7-8 pS, whereas fully edited GluR2 channels had very low conductances of approximately 300 fS (estimated from noise analysis). Additionally, the flip splice variant of GluR4 conferred agonist-dependent conductance properties reminiscent of those found for a subset of AMPA receptors in cultured cerebellar granule cells. These results provide a description of the single-channel properties of certain recombinant AMPA receptors and suggest that the single-channel conductance may be determined by the expression of edited GluR2 subunits in neurons.
Figures


References
-
- Barker JL, Mathers DA. GABA analogues activate channels of different duration on cultures mouse spinal neurons. Science. 1981;212:358–361. - PubMed
-
- Bowie D, Mayer M. Inward rectification of both AMPA and kainate subtype glutamate receptors generated by polyamine-mediated ion channel block. Neuron. 1995;15:453–462. - PubMed
-
- Burnashev N, Monyer H, Seeburg PH, Sakmann B. Divalent ion permeability of AMPA receptor channels is dominated by the edited form of a single subunit. Neuron. 1992;8:189–198. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
