The role of synaptic and voltage-gated currents in the control of Purkinje cell spiking: a modeling study
- PMID: 8987739
- PMCID: PMC6793698
- DOI: 10.1523/JNEUROSCI.17-01-00091.1997
The role of synaptic and voltage-gated currents in the control of Purkinje cell spiking: a modeling study
Abstract
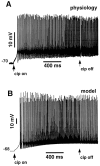
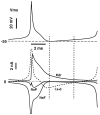
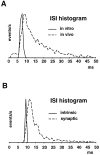
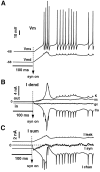
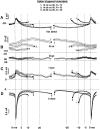
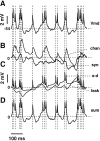
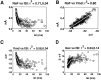
We have used a realistic computer model to examine interactions between synaptic and intrinsic voltage-gated currents during somatic spiking in cerebellar Purkinje cells. We have shown previously that this model generates realistic in vivo patterns of somatic spiking in the presence of continuous background excitatory and inhibitory input (). In the present study, we analyzed the flow of synaptic and intrinsic currents across the dendritic membrane and the interaction between the soma and dendrite underlying this spiking behavior. This analysis revealed that: (1) dendritic inward current flow was dominated by a noninactivating P-type calcium current, resulting in a continuous level of depolarization; (2) the mean level of this depolarization was controlled by the mean rate of excitatory and inhibitory synaptic input; (3) the synaptic control involved a voltage-clamping mechanism exerted by changes of synaptic driving force at different membrane potentials; (4) the resulting total current through excitatory and inhibitory synapses was near-zero, with a small outward bias opposing the P-type calcium current; (5) overall, the dendrite acted as a variable current sink with respect to the soma, slowing down intrinsic inward currents in the soma; (6) the somato-dendritic current showed important phasic changes during each spike cycle; and (7) the precise timing of somatic spikes was the result of complex interactions between somatic and dendritic currents that did not directly reflect the timing of synaptic input. These modeling results suggest that Purkinje cells act quite differently from simple summation devices, as has been assumed previously in most models of cerebellar function. Specific physiologically testable predictions are discussed.
Figures




References
-
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61.
-
- Bell AJ, Mainen ZF, Tsodyks M, Sejnowski TJ. Balancing of conductances may explain irregular cortical spiking. INC Technical Report no. INC-9502. University of California; San Diego: 1994.
-
- Bower JM, Woolston DC. Congruence of spatial organization of tactile projections to granule cell and Purkinje cell layers of cerebellar hemispheres of the albino rat: vertical organization of cerebellar cortex. J Neurophysiol. 1983;49:745–766. - PubMed
-
- Bower JM (1996) Is the cerebellum sensory for motor’s sake, or motor for sensory’s sake: the view from the whiskers of a rat. Prog Brain Res, in press. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
