Postnatal development of corticospinal projections from motor cortex to the cervical enlargement in the macaque monkey
- PMID: 8987753
- PMCID: PMC6793701
- DOI: 10.1523/JNEUROSCI.17-01-00251.1997
Postnatal development of corticospinal projections from motor cortex to the cervical enlargement in the macaque monkey
Abstract
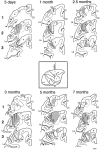
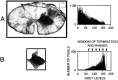
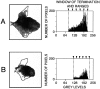
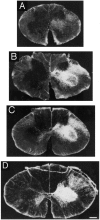
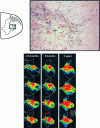
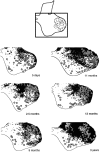
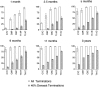
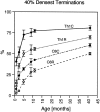
The postnatal development of corticospinal projections was investigated in 11 macaques by means of the anterograde transport of wheat germ agglutin-horseradish peroxidase injected into the primary motor cortex hand area. Although the fibers of the corticospinal tract reached all levels of the spinal cord white matter at birth, their penetration into the gray matter was far from complete. At birth, as in the adult, corticospinal projections were distributed to the same regions of the intermediate zone, although they showed marked increases in density during the first 5 months. The unique feature of the primate corticospinal tract, namely direct cortico-motoneuronal projections to the spinal motor nuclei innervating hand muscles, was not present to a significant extent at birth. The density of these cortico-motoneuronal projections increased rapidly during the first 5 months, followed by a protracted period extending into the second year of life. The densest corticospinal terminations occupied only 40% of the hand motor nuclei in the first thoracic segment at 1 month, 73% at 5 months, and 75.5% at 3 years. A caudo-rostral gradient of termination density within the hand motor nuclei was present throughout development and persisted into the adult. As a consequence, the more caudal the segment within the cervical enlargement, the earlier the adult pattern of projection density was reached. No transitory corticospinal projections were found. The continuous postnatal expansion of cortico-motoneuronal projections to hand motor nuclei in primates is in marked contrast to the retraction of exuberant projections that characterizes the development of other sensory and motor pathways in subprimates.
Figures
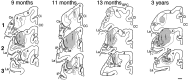
References
-
- Alisky JM, Swink TD, Tolbert DL. The postnatal spatial and temporal development of corticospinal projections in the cat. Exp Brain Res. 1992;88:265–276. - PubMed
-
- Armand J, Holstege G, Kuypers HGJM. Differential corticospinal projections in the cat: an autoradiographic tracing study. Brain Res. 1985;343:351–355. - PubMed
-
- Armand J, Edgley SA, Lemon RN, Olivier E. Protracted postnatal development of corticospinal projections from the primary motor cortex to hand motoneurones in the macaque monkey. Exp Brain Res. 1994;101:178–182. - PubMed
-
- Armand J, Olivier E, Edgley S, Lemon RN. The structure and function of the developing corticospinal tract: some key issues. In: Wing AH, Haggard P, Flanagan JR, editors. Hand and brain. The neurophysiology and psychology of hand movements. Academic; San Diego: 1996. pp. 125–145.
-
- Babalian A, Liang F, Rouiller EM. Cortical influences on cervical motoneurons in the rat: recordings of synaptic responses from motoneurons and compound action potential from corticospinal axons. J Neurosci Res. 1993;16:301–310. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
