Nerve growth factor induces apoptosis in human medulloblastoma cell lines that express TrkA receptors
- PMID: 8987776
- PMCID: PMC6573234
- DOI: 10.1523/JNEUROSCI.17-02-00530.1997
Nerve growth factor induces apoptosis in human medulloblastoma cell lines that express TrkA receptors
Abstract
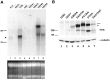
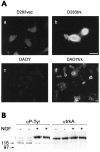
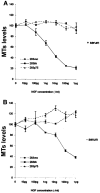
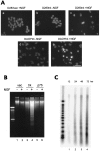
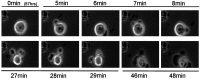
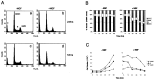
Neurotrophins act through their cognate receptors to promote the differentiation and/or survival of neuronal progenitor cells, immature neurons, and other cells. Here, we examined the effects of nerve growth factor (NGF) and its cognate receptor (Trk or TrkA) on the survival of a common childhood brain tumor, i.e., medulloblastoma, a tumor that resembles CNS neuroepithelial progenitor cells. To do this, we engineered two human medulloblastoma cell lines (i.e., D283MED and DAOY cells) to express human TrkA using a retroviral expression vector. Surprisingly, NGF-treated medulloblastoma cells expressing the TrkA receptor (D283trk and DAOYtrk cells) grown in the presence or absence of serum underwent massive apoptosis, but similar treatment did not induce apoptosis in wild-type uninfected cells, cells expressing an empty vector, or cells expressing the TrkC receptor. Furthermore, D283MED cells engineered to express the human p75 NGF receptor (D283p75) also did not undergo apoptosis. Significantly, NGF-induced apoptosis in D283trk and DAOYtrk cells can be inhibited by anti-NGF antibodies and by K-252a, an inhibitor of TrkA tyrosine phosphorylation and mimicked by high concentrations of NT3. Because NGF treatment primarily eliminated D283trk cells from the S phase of the cell cycle, this form of NGF-mediated apoptosis is cell cycle-dependent. These findings suggest that a NGF/TrkA signal transduction pathway could activate apoptotic cell death programs in CNS neuroepithelial progenitor cells and in childhood brain tumors.
Figures


References
-
- Baker DL, Molenaar WM, Trojanowski JQ, Evans AE, Ross AH, Rorke LB, Packer R, Lee VM-Y, Pleasure D. Nerve growth factor receptor expression in peripheral and central primitive neuroectodermal tumors, other pediatric brain tumors and during development of the adrenal gland. Am J Pathol. 1991;139:115–122. - PMC - PubMed
-
- Barbacid M. Neurotrophic factors and their receptors. Curr Opin Cell Biol. 1995;7:148–155. - PubMed
-
- Barker PA, Lomen-Hoerth C, Gensch EM, Meakin SO, Glass DJ, Shooter EM. Tissue-specific alternative splicing generates 2 isoforms of the trk receptor. J Biol Chem. 1993;268:15150–15157. - PubMed
-
- Becker LE, Hinton D. Primitive neuroectodermal tumors of the central nervous system. Hum Pathol. 1983;14:538–550. - PubMed
-
- Berg MM, Sternberg DW, Parada LF, Chao MV. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem. 1992;267:13–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
