Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus
- PMID: 8987780
- PMCID: PMC6573224
- DOI: 10.1523/JNEUROSCI.17-02-00576.1997
Role of Ca2+ ions in nicotinic facilitation of GABA release in mouse thalamus
Abstract
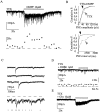
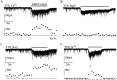
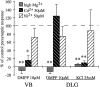
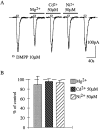
Presynaptic nicotinic acetylcholine receptors (nAChRs) are present in many regions of the brain and potentially serve as targets for the pharmacological action of nicotine in vivo. To investigate their mechanism of action, we performed patch-clamp recordings in relay neurons from slices of thalamus sensory nuclei. In these nuclei, nAChR activation facilitated the release of the inhibitory neurotransmitter GABA. Micromolar concentrations of nicotinic agonists increased the frequency of miniature GABAergic synaptic currents and decreased the failure rate of evoked synaptic currents. These actions of nicotinic agonists were not observed in knock-out mice lacking the beta 2 nAChR subunit gene. Nicotinic effects were dependent on extracellular calcium ions, and they persisted when calcium was replaced by strontium or barium but not by magnesium. Furthermore, in high extracellular calcium concentrations, nicotinic agonists evoked an increase in spontaneous release lasting for minutes after removal of the agonist. This supports the view that presynaptic nAChRs facilitate the release of neurotransmitter by increasing the calcium concentrations in presynaptic nerve endings. With use of cadmium and nickel ions as selective blockers, it was found that in different sensory nuclei the presynaptic influx of calcium could result either from the activation of voltage-dependent calcium channels or from a direct influx through nAChR channels. Finally, we propose that the nicotinic facilitation of GABAergic transmission may contribute to the increase of signal-to-noise ratio observed in the thalamus in vivo during arousal.
Figures




References
-
- Alkondon M, Albuquerque EX. Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. I. Pharmacological and functional evidence for distinct structural subtypes. J Pharmacol Exp Ther. 1993;265:1455–1473. - PubMed
-
- Ankri N, Legendre P, Faber DS, Korn H. Automatic detection of spontaneous synaptic responses in central neurons. J Neurosci Methods. 1994;52:87–100. - PubMed
-
- Britto L, Keyser K, Lindstrom J, Karten H. Immunohistochemical localization of nicotinic acetylcholine receptor subunits in the mesencephalon and diencephalon of the chick (Gallus gallus). J Comp Neurol. 1992;317:325–340. - PubMed
-
- Clarke PBS, Pert A. Autoradiographic evidence for nicotine receptors on nigrostriatal and mesolimbic dopaminergic neurons. Brain Res. 1985;348:355–358. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
