Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor
- PMID: 8987785
- PMCID: PMC6573228
- DOI: 10.1523/JNEUROSCI.17-02-00625.1997
Bicuculline and gabazine are allosteric inhibitors of channel opening of the GABAA receptor
Abstract
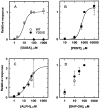
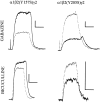
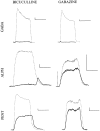
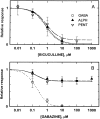
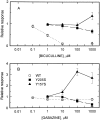
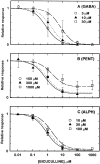
Anesthetic drugs are known to interact with GABAA receptors, both to potentiate the effects of low concentrations of GABA and to directly gate open the ion channel in the absence of GABA; however, the site(s) involved in direct gating by these drugs is not known. We have studied the ability of alphaxalone (an anesthetic steroid) and pentobarbital (an anesthetic barbiturate) to directly activate recombinant GABAA receptors containing the alpha 1, beta 2, and gamma 2L subunits. Steroid gating was not affected when either of two mutated beta 2 subunits [beta 2 (Y157S) and beta 2 (Y205S)] are incorporated into the receptors, although these subunits greatly reduce the affinity of GABA binding. These observations indicate that steroid binding and subsequent channel gating do not require these particular residues, as already shown for barbiturates. Bicuculline or gabazine (two competitive antagonists of GABA binding) reduced the currents elicited by alphaxalone and pentobarbital from wild-type GABAA receptors; however, gabazine produced only a partial block of response pentobarbital or alphaxalone, and bicuculline only partially blocked responses to pentobarbital. These observations indicate that the blockers do not compete with alphaxalone or pentobarbital for a single class of sites on the GABAA receptor. Finally, at receptors containing alpha 1 beta 2 (Y157S) gamma 2L subunits, both bicuculline and gabazine showed weak agonist activity and actually potentiated responses to alphaxalone. These observations indicate that the blocking drugs can produce allosteric changes in GABAA receptors, at least those containing this mutated beta 2 subunit. We conclude that the sites for binding steroids and barbiturates do not overlap with the GABA-binding site. Furthermore, neither gabazine nor bicuculline competes for binding at the steroid or barbiturate sites. The data are consistent with a model in which both gabazine and bicuculline act as allosteric inhibitors of channel opening for the GABAA receptor after binding to the GABA-binding site.
Figures

References
-
- Amin J, Weiss DS. GABAA receptor needs two homologous domains of the β-subunit for activation by GABA but not by pentobarbital. Nature. 1993;366:565–569. - PubMed
-
- Arakawa O, Nakahiro M, Narahashi T. Chloride current induced by alcohols in rat dorsal root ganglion neurons. Brain Res. 1992;578:275–281. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
