Increased probability of GABA release during withdrawal from morphine
- PMID: 8987801
- PMCID: PMC6573250
- DOI: 10.1523/JNEUROSCI.17-02-00796.1997
Increased probability of GABA release during withdrawal from morphine
Abstract
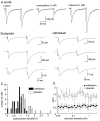
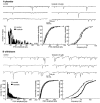
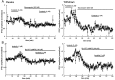
Opioid receptors located on interneurons in the ventral tegmental area (VTA) inhibit GABA(A)-mediated synaptic transmission to dopamine projection neurons. The resulting disinhibition of dopamine cells in the VTA is thought to play a pivotal role in drug abuse; however, little is known about how this GABAA synapse is affected after chronic morphine treatment. The regulation of GABA release during acute withdrawal from morphine was studied in slices from animals treated for 6-7 d with morphine. Slices containing the VTA were prepared and maintained in morphine-free solutions, and GABAA IPSCs were recorded from dopamine cells. The amplitude of evoked IPSCs and the frequency of spontaneous miniature IPSCs measured in slices from morphine-treated guinea pigs were greater than placebo-treated controls. In addition, activation of adenylyl cyclase, with forskolin, and cAMP-dependent protein kinase, with Sp-cAMPS, caused a larger increase in IPSCs in slices from morphine-treated animals. Conversely, the kinase inhibitors staurosporine and Rp-CPT-cAMPS decreased GABA IPSCs to a greater extent after drug treatment. The results indicate that the probability of GABA release was increased during withdrawal from chronic morphine treatment and that this effect resulted from an upregulation of the cAMP-dependent cascade. Increased transmitter release from opioid-sensitive synapses during acute withdrawal may be one adaptive mechanism that results from prolonged morphine treatment.
Figures



References
-
- Aghajanian GK. Tolerance of locus coeruleus neurons to morphine and suppression of withdrawal response by clonidine. Nature. 1978;276:186–187. - PubMed
-
- Andrade R, Vandermaelen CP, Aghajanian GK. Morphine tolerance and dependence in the locus coeruleus: single cell studies in brain slices. Eur J Pharmacol. 1983;91:161–165. - PubMed
-
- Bonci A, Williams JT. A common mechanism mediates long-term changes in synaptic transmission after chronic cocaine and morphine. Neuron. 1996;16:631–639. - PubMed
-
- Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981;28:551–555. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
