Differential localization of delta glutamate receptors in the rat cerebellum: coexpression with AMPA receptors in parallel fiber-spine synapses and absence from climbing fiber-spine synapses
- PMID: 8987804
- PMCID: PMC6573242
- DOI: 10.1523/JNEUROSCI.17-02-00834.1997
Differential localization of delta glutamate receptors in the rat cerebellum: coexpression with AMPA receptors in parallel fiber-spine synapses and absence from climbing fiber-spine synapses
Abstract
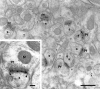
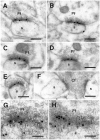
The delta 2 glutamate receptors are prominently expressed in Purkinje cells and are thought to play a key role in the induction of cerebellar long-term depression. The synaptic and subsynaptic localization of delta receptors in rat cerebellar cortex was investigated with sensitive and high-resolution immunogold procedures. After postembedding incubation with an antibody raised to a C-terminal peptide of delta 2, high gold particle densities occurred in all parallel fiber synapses with Purkinje cell dendritic spines, whereas other synapses were consistently devoid of labeling. Among the types of immunonegative synapse were climbing fiber synapses with spines and parallel fiber synapses with dendritic stems of interneurons. At the parallel fiber-spine synapse, gold particles signaling delta receptors were restricted to the postsynaptic specialization. By the use of double labeling with two different gold particle sizes, it was shown that delta and AMPA GluR2/3 receptors were colocalized along the entire extent of the postsynaptic specialization without forming separate domains. The distribution of gold particles representing delta receptors was consistent with a cytoplasmic localization of the C terminus and an absence of a significant presynaptic pool of receptor molecules. The present data suggest that the delta 2 receptors are targeted selectively to a subset of Purkinje cell spines and that they are coexpressed with ionotropic receptors in the postsynaptic specialization. This arrangement could allow for a direct interaction between the two classes of receptor.
Figures



References
-
- Araki K, Meguro H, Kushiya E, Takayama C, Inoue Y, Mishina M. Selective expression of the glutamate receptor channel delta 2 subunit in cerebellar Purkinje cells. Biochem Biophys Res Commun. 1993;197:1267–1276. - PubMed
-
- Baude A, Nusser Z, Roberts JDB, Mulvihill E, McIlhinney RAJ, Somogyi P. The metabotropic glutamate receptor (mGluR1α) is concentrated at perisynaptic membrane of neuronal subpopulations as detected by immunogold reaction. Neuron. 1993;11:771–787. - PubMed
-
- Baude A, Nusser Z, Molnár E, McIlhinney RAJ, Somogyi P. High-resolution immunogold localization of AMPA-type glutamate receptor subunits at synaptic and nonsynaptic sites in the rat hippocampus. Neuroscience. 1995;69:1031–1055. - PubMed
-
- Bennet JA, Dingledine R. Topology profile for a glutamate receptor: three transmembrane domains and a channel-lining reentrant membrane loop. Neuron. 1995;14:373–384. - PubMed
-
- Chaudhry FA, Lehre KP, van Lookeren Campagne M, Ottersen OP, Danbolt NC, Storm-Mathisen J. Glutamate transporters in glial plasma membranes: highly differentiated localizations revealed by quantitative ultrastructural immunocytochemistry. Neuron. 1995;15:711–720. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
