Translocation of RNA granules in living neurons
- PMID: 8987809
- PMCID: PMC6579227
- DOI: 10.1523/JNEUROSCI.16-24-07812.1996
Translocation of RNA granules in living neurons
Abstract
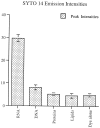
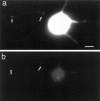
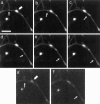
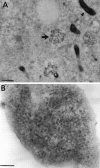
Sorting of RNAs to specific subcellular loci occurs in diverse settings from fly oocytes to mammalian neurons. Using the membrane-permeable nucleic acid stain SYTO 14, we directly visualized the translocation of endogenous RNA in living cells. Labeled RNA was distributed nonrandomly as discrete granules in neuronal processes. The labeled granules colocalized with poly(A+) mRNA, with the 60S ribosomal subunit, and with elongation factor 1alpha, suggesting that granules represent a translational unit. A subset of labeled granules colocalized with beta-actin mRNA. Correlative light and electron microscopy indicated that the fluorescent granules corresponded to clusters of ribosomes at the ultrastructural level. Poststaining of sections with heavy metals confirmed the presence of ribosomes within these granules. In living neurons, a subpopulation of RNA granules was motile during the observation period. They moved at an average rate of 0.1 microm/sec. In young cultures their movements were exclusively anterograde, but after 7 d in culture, one-half of the motile granules moved in the retrograde direction. Granules in neurites were delocalized after treatment with microtubule-disrupting drugs. These results raise the possibility of a cellular trafficking system for the targeting of RNA in neurons.
Figures



References
-
- Atkinson S, Doberstein SK, Pollard TD. Moving off the beaten track. Curr Biol. 1992;2:326–328. - PubMed
-
- Banker GA, Waxman AB. Hippocampal neurons generate natural shapes in cell culture. In: Lasek RJ, Black MM, editors. Studies of the intrinsic determinants of neuronal form and function. Liss; New York: 1988. pp. 61–82.
-
- Barbarese E, Koppel DE, Deutscher MP, Smith CL, Ainger K, Morgan F, Carson JH. Protein translation components are colocalized in granules in oligodendrocytes. J Cell Sci. 1995;108:2781–2790. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
