Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1-40)
- PMID: 8987819
- PMCID: PMC6579235
- DOI: 10.1523/JNEUROSCI.16-24-07910.1996
Matrix metalloproteinase-9 (MMP-9) is synthesized in neurons of the human hippocampus and is capable of degrading the amyloid-beta peptide (1-40)
Abstract
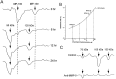
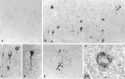
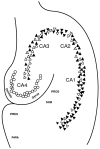
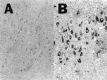
We reported earlier that the levels of Ca2+-dependent metalloproteinases are increased in Alzheimer's disease (AD) specimens, relative to control specimens. Here we show that these enzymes are forms of the matrix metalloproteinase MMP-9 (EC3.4.24. 35) and are expressed in the human hippocampus. Affinity-purified antibodies to MMP-9 labeled pyramidal neurons, but not granular neurons or glial cells. MMP-9 mRNA is expressed in pyramidal neurons, as determined with digoxigenin-labeled MMP-9 riboprobes, and the presence of this mRNA is confirmed with reverse transcriptase PCR. The cellular distribution of MMP-9 is altered in AD because 76% of the total 100 kDa enzyme activity is found in the soluble fraction of control specimens, whereas only 51% is detectable in the same fraction from AD specimens. The accumulated 100 kDa enzyme from AD brain is latent and can be converted to an active form with aminophenylmercuric acetate. MMP-9 also is detected in close proximity to extracellular amyloid plaques. Because a major constituent of plaques is the 4 kDa beta-amyloid peptide, synthetic Abeta1-40 was incubated with activated MMP-9. The enzyme cleaves the peptide at several sites, predominantly at Leu34-Met35 within the membrane-spanning domain. These results establish that neurons have the capacity to synthesize MMP-9, which, on activation, may degrade extracellular substrates such as beta-amyloid. Because the latent form of MMP-9 accumulates in AD brain, it is hypothesized that the lack of enzyme activation contributes to the accumulation of insoluble beta-amyloid peptides in plaques.
Figures

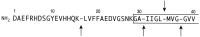
References
-
- Abraham CR, Selkoe DJ, Potter H. Immunochemical identification of the serine proteinase inhibitor, alpha-1-antichymotrypsin, in the brain amyloid deposit of Alzheimer’s disease. Cell. 1988;52:487–501. - PubMed
-
- Aisen PS, Davis KL. Inflammatory mechanisms in Alzheimer’s disease: implications for therapy. Am J Psychiatry. 1994;151:1105–1113. - PubMed
-
- Backstrom JR, Tökés ZA. The 84 kDa form of human matrix metalloproteinase-9 (MMP-9) degrades substance P and gelatin. J Neurochem. 1995;64:1312–1318. - PubMed
-
- Backstrom JR, Miller CA, Tökés ZA. Characterization of neutral proteinases from Alzheimer-affected and control brain specimens: identification of calcium-dependent metalloproteinases from the hippocampus. J Neurochem. 1992;58:983–992. - PubMed
-
- Breitner JCS, Gau BA, Welsh KA, Plassman BL, McDonald WM, Helms MJ, Anthony JC. Inverse association of anti-inflammatory treatments and Alzheimer’s disease: initial results of a co-twin control study. Neurology. 1994;44:227–232. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
