Cell type-specific sorting of neuropeptides: a mechanism to modulate peptide composition of large dense-core vesicles
- PMID: 8987821
- PMCID: PMC6579229
- DOI: 10.1523/JNEUROSCI.16-24-07930.1996
Cell type-specific sorting of neuropeptides: a mechanism to modulate peptide composition of large dense-core vesicles
Abstract
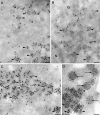
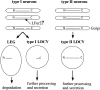
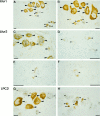
The CNS of Lymnaea stagnalis contains two populations of egg-laying hormone (ELH)-producing neurons that differ in size and topology. In type I neurons, all peptides located C-terminally from the cleavage site Arg-Ser-Arg-Arg180-183 are sorted into secretory large dense-core vesicles (LDCV), whereas N-terminal-located peptides accumulate in a distinct type of vesicle, the large electrondense granule (LEG). Via immunoelectron microscopy, we now show that the second population of ELH-producing neurons, type II neurons, lack LEG and incorporate all proELH-derived peptides into LDCV. This finding provides the first example of a cell type-specific sorting of neuropeptides into LDCV. Furthermore, we provide evidence that LEG are formed through a differential condensation process in the trans-Golgi network and that these bodies are ultimately degraded. Analysis of the endoprotease composition of the two types of proELH-producing neurons suggests that the formation of LEG, and consequently the retention of N-terminal peptides from the secretory pathway, requires the action of a furin-like protein.
Figures






References
-
- Barka R, Anderson PJ. Histochemical methods for acid phosphatase using hexazonium pararosanilin as coupler. J Histochem Cytochem. 1962;10:741–753.
-
- Chun JY, Korner J, Kreiner T, Scheller RH, Axel R. The function and differential sorting of a family of Aplysia prohormone processing enzymes. Neuron. 1994;12:831–844. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
