Influence of cocaine on the JAK-STAT pathway in the mesolimbic dopamine system
- PMID: 8987828
- PMCID: PMC6579209
- DOI: 10.1523/JNEUROSCI.16-24-08019.1996
Influence of cocaine on the JAK-STAT pathway in the mesolimbic dopamine system
Abstract
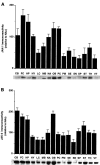
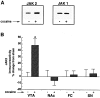
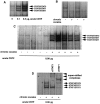
Chronic exposure to cocaine produces characteristic biochemical adaptations within the rat ventral tegmental area (VTA), a brain region rich in dopaminergic neurons implicated in the reinforcing and locomotor-activating properties of cocaine. Some of these changes are mimicked by chronic ciliary neurotrophic factor (CNTF) infusions into the same brain area. We show in this study that chronic cocaine treatment regulates the signal transduction pathway used by CNTF specifically in the VTA. There is an increase in immunoreactivity of Janus kinase (JAK2), a CNTF-regulated protein tyrosine kinase, in the VTA after chronic but not acute cocaine administration. This increase is not seen in the nearby substantia nigra or several other brain regions studied. Furthermore, this increase in JAK2 is not seen after chronic administration of other psychotropic drugs and was not observed for JAK1. The increase in JAK2 levels is associated with an increased responsiveness of the system to acute CNTF infusion into the VTA, as measured by induction in this brain region of signal transducers and activators of transcription (STAT) DNA binding activity and of Fos-like proteins, two known functional endpoints of JAK activation. Double-labeling immunohistochemical studies show that JAK2 immunoreactivity in the VTA is enriched in dopaminergic and nondopaminergic cells, both of which exhibit increased JAK2 immunoreactivity after chronic cocaine treatment. These findings suggest a scheme whereby some of the effects of chronic cocaine on VTA dopaminergic neurons are mediated directly by regulation of the JAK-STAT pathway in these cells, as well as perhaps indirectly by regulation of this pathway in nondopaminergic cells.
Figures


References
-
- Angel P, Karin M. The role of Jun, Fos and AP-1 complex in cell-proliferation and transformation. Biochim Biophys Acta. 1991;1072:129–157. - PubMed
-
- Beitner-Johnson D, Nestler EJ. Morphine and cocaine exert common chronic actions on tyrosine hydroxylase in dopaminergic brain reward regions. J Neurochem. 1991;57:344–347. - PubMed
-
- Beitner-Johnson D, Guitart X, Nestler EJ. Glial fibrillary acidic protein and the mesolimbic dopamine system: regulation by chronic morphine and Lewis-Fischer strain differences in the rat ventral tegmental area. J Neurochem. 1993;61:1766–1773. - PubMed
-
- Berhow MT, Russell DS, Terwilliger RZ, Beitner-Johnson D, Self DW, Lindsay RM, Nestler EJ. Influence of neurotrophic factors on morphine- and cocaine-induced biochemical changes in the mesolimbic dopamine system. Neuroscience. 1995;68:969–979. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
