Local control of leg movements and motor patterns during grooming in locusts
- PMID: 8987832
- PMCID: PMC6579221
- DOI: 10.1523/JNEUROSCI.16-24-08067.1996
Local control of leg movements and motor patterns during grooming in locusts
Abstract
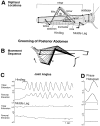
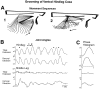
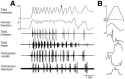
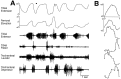
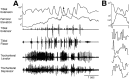
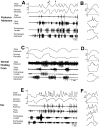
This study demonstrates that the thoracic and abdominal nervous system of locusts is sufficient to mediate several site-specific and distinct grooming leg movements. Locusts can use a hindleg or middle leg to groom at least four ipsilateral thoracic and abdominal sites, without input from the brain, subesophageal ganglion, or prothoracic ganglion. The hindleg is used to groom the posterior abdomen, the ventral or posterior hindleg coxa, and the ear; the middle leg is used to groom the anterior hindleg coxa. Grooming movements are often rhythmic and display site-specific intralimb coordination patterns. During grooming of the posterior abdomen or ventral hindleg coxa, for example, hindleg tibial extension occurs nearly simultaneously with femur elevation, in contrast with locust hindleg movements during walking. Electromyographic (EMG) recordings during these movements show that rhythmic bursts of tibial extensor activity occur nearly in-phase with those of trochanteral levators, in contrast to hindleg EMGs during walking. During grooming of the ear, hindleg tibial extension/flexion and tibial extensor/flexor muscle bursts can occur independently of the femur elevation/depression and trochanteral levator/depressor muscle bursts, suggesting that the neural modules controlling tibial and femoral movements can be uncoupled during this behavior. Tibial extension can occur before, or even in the absence of, tibial extensor muscle activity, suggesting that spring-like properties of the leg and energy transfer from femur motion may play important roles in such leg movements. Adjacent legs sometime show coordinated femur movement during grooming with one hindleg, suggesting that grooming may also involve interlimb coordination.
Figures



References
-
- Bässler U. On the definition of central pattern generator and its sensory control. Biol Cybern. 1986;54:65–69.
-
- Bässler U. The walking- (and searching-) pattern generator of stick insects, a modular system composed of reflex chains and endogenous oscillators. Biol Cybern. 1993;69:305–317.
-
- Berkowitz A, Laurent G. Central generation of directed limb movements in locusts. Soc Neurosci Abstr. 1995;21:1764.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
