Central generation of grooming motor patterns and interlimb coordination in locusts
- PMID: 8987833
- PMCID: PMC6579234
- DOI: 10.1523/JNEUROSCI.16-24-08079.1996
Central generation of grooming motor patterns and interlimb coordination in locusts
Abstract
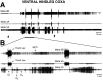
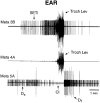
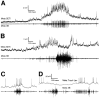
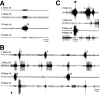
Coordinated bursts of leg motoneuron activity were evoked in locusts with deefferented legs by tactile stimulation of sites that evoke grooming behavior. This suggests that insect thoracic ganglia contain central pattern generators for directed leg movements. Motoneuron recordings were made from metathoracic and mesothoracic nerves, after eliminating all leg motor innervation, as well as all input from the brain, subesophageal ganglion, and prothoracic ganglion. Strong, brief trochanteral levator motoneuron bursts occurred, together with silence of the slow and fast trochanteral depressor motoneurons and activation of the common inhibitor motoneuron. The metathoracic slow tibial extensor motoneuron was active in a pattern distinct from its activity during walking or during rhythms evoked by the muscarinic agonist pilocarpine. Preparations in which the metathoracic ganglion was isolated from all other ganglia could still produce fictive motor patterns in response to tactile stimulation of metathoracic locations. Bursts of trochanteral levator and depressor motoneurons were clearly coordinated between the left and right metathoracic hemiganglia and also between the mesothoracic and the ipsilateral metathoracic ganglia. These data provide clear evidence for centrally generated interlimb coordination in an insect.
Figures





References
-
- Arshavsky YI, Gelfand IM, Orlovsky GN, Pavlova GA. Messages conveyed by spinocerebellar pathways during scratching in the cat. II. Activity of neurons of the ventral spinocerebellar tract. Brain Res. 1978;151:493–506. - PubMed
-
- Barajon I, Gossard JP, Hultborn H. Induction of fos expression by activity in the spinal rhythm generator for scratching. Brain Res. 1992;588:168–172. - PubMed
-
- Bässler U. On the definition of central pattern generator and its sensory control. Biol Cybern. 1986;54:65–69.
-
- Bässler U. The walking- (and searching-) pattern generator of stick insects, a modular system composed of reflex chains and endogenous oscillators. Biol Cybern. 1993;69:305–317.
-
- Bässler U, Wegner U. Motor output of the denervated thoracic ventral nerve cord in the stick insect Carausius morosus. J Exp Biol. 1983;105:127–145.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
