Release of peptide cotransmitters in Aplysia: regulation and functional implications
- PMID: 8987835
- PMCID: PMC6579220
- DOI: 10.1523/JNEUROSCI.16-24-08105.1996
Release of peptide cotransmitters in Aplysia: regulation and functional implications
Abstract
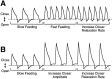
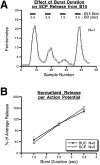
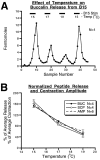
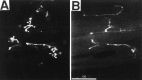
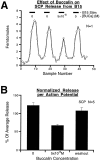
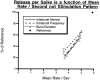
To gain insights into the physiological role of cotransmission, we measured peptide release from cell B15, a motorneuron that utilizes ACh as its primary transmitter but also contains putative peptide cotransmitters, the small cardioactive peptides (SCPs) and the buccalins (BUCs). All stimulation parameters used were in the range in which B15 fires in freely moving animals. We stimulated neuron B15 in bursts and systematically varied the interburst interval, the intraburst frequency, and burst duration. Both peptides were preferentially released when B15 was stimulated at higher intra- or interburst frequencies or with longer burst durations. Across stimulation patterns, the amount of peptide released depended on the mean frequency of stimulation and was independent of the specific pattern of stimulation. The parameters of stimulation that produce a larger release of peptides correspond to those that evoke larger contractions. Large and frequent contractions are likely to fuse or summate, thus disrupting the rhythmic behavior mediated by the muscle innervated by motorneuron B15. Because the combined effect of the SCPs and BUCs is to accelerate the relaxation and shorten the duration of muscle contractions, these peptides reduce the probability of the disruptive fusion or summation of muscle contractions. Because these cotransmitters regulate an aspect of muscle contractions that is not controlled by acetylcholine (ACh), the primary transmitter of B15, we suggest that peptides and ACh form parallel but functionally distinct lines of transmission at the neuromuscular junction. Both types of transmission may be necessary to ensure that behavior remains efficient over a wide range of conditions.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
