Spontaneous discharge and peripherally evoked orofacial responses of trigemino-thalamic tract neurons during wakefulness and sleep
- PMID: 8987840
- PMCID: PMC6579215
- DOI: 10.1523/JNEUROSCI.16-24-08149.1996
Spontaneous discharge and peripherally evoked orofacial responses of trigemino-thalamic tract neurons during wakefulness and sleep
Abstract
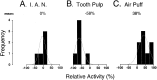
In the present study, ongoing and evoked activity of antidromically identified trigemino-thalamic tract (TGT) neurons was examined over the sleep-wake cycle in cats. There was no difference in the mean spike discharge rate of TGT neurons when quiet sleep (QS) and active sleep (AS) were compared with wakefulness (W). However, tooth pulp-evoked responses of TGT neurons were decreased during AS when compared to W. Conversely, the responses of TGT neurons to air puff activation of facial hair mechanoreceptors reciprocally increased during AS when compared to W. The present data demonstrate that ascending sensory information emanating from distinct orofacial areas is differentially modified during the behavioral state of AS. Specifically, the results obtained suggest that during AS, sensory information arising from hair mechanoreceptors is enhanced, whereas information arising from tooth pulp afferents is suppressed. These data may provide functional evidence for an AS-related gate control mechanism of sensory outflow to higher brain centers.
Figures








References
-
- Amano N, Hu JW, Sessle BJ. Responses of neurons in feline trigeminal subnucleus caudalis (medullary dorsal horn) to cutaneous, intraoral, and muscle afferent stimuli. J Neurophysiol. 1986;55:227–243. - PubMed
-
- Azerad J, Woda A, Albe-Fessard D. Physiological properties of neurons in different parts of the cat sensory complex. Brain Res. 1982;246:7–21. - PubMed
-
- Boissonade FM, Matthews B. Responses of trigeminal brain stem neurons and the digastric muscle to tooth-pulp stimulation in awake cats. J Neurophysiol. 1993;69:174–186. - PubMed
-
- Burton H, Craig AD. Distribution of trigeminothalamic projection cells in cat and monkey. Brain Res. 1979;161:515–521. - PubMed
-
- Bushnell MD, Duncan GH, Dubner R, He LF. Activity of trigeminothalamic neurons in medullary dorsal horn of awake monkeys trained in a thermal discrimination task. J Neurophysiol. 1984;52:170–187. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
