Electronic clinical trial protocol distribution via the World-Wide Web: a prototype for reducing costs and errors, improving accrual, and saving trees
- PMID: 8988471
- PMCID: PMC61195
- DOI: 10.1093/jamia/4.1.25
Electronic clinical trial protocol distribution via the World-Wide Web: a prototype for reducing costs and errors, improving accrual, and saving trees
Abstract
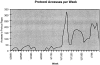
Clinical trials today typically are inefficient, paper-based operations. Poor community physician awareness of available trials and difficult referral mechanisms also contribute to poor accrual. The Physicians Research Network (PRN) web was developed for more efficient trial protocol distribution and eligibility inquiries. The Medical University of South Carolina's Hollings Cancer Center trials program and two community oncology practices served as a testbed. In 581 man-hours over 18 months, 147 protocols were loaded into PRN. The trials program eliminated all protocol hardcopies except the masters, reduced photocopier use 59%, and saved 1.0 full-time equivalents (FTE), but 1.0 FTE was needed to manage PRN. There were no known security breaches, downtime, or content-related problems. Therefore, PRN is a paperless, user-preferred, reliable, secure method for distributing protocols and reducing distribution errors and delays because only a single copy of each protocol is maintained. Furthermore, PRN is being extended to serve other aspects of trial operations.
Figures


References
-
- Jenkins J, Hubbard S. History of clinical trials. Semin Oncol Nurs. 1991;7:228 -34. - PubMed
-
- Friedman L. The NHLBI model: a 25 year history. Stat Med. 1993;12:425 -31. - PubMed
-
- Mansour EG. Barriers to clinical trials. Part III: Knowledge and attitudes of health care providers. Cancer.1994. ;74:2672 -5. - PubMed
-
- Fisher B. On clinical trial participation. J Clin Oncol. 1991;9:1927 -30. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

