Spare the rod, spoil the regulation: necessity for a myosin rod
- PMID: 8990159
- PMCID: PMC19234
- DOI: 10.1073/pnas.94.1.48
Spare the rod, spoil the regulation: necessity for a myosin rod
Abstract
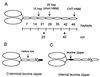
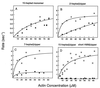
Regulation of a variety of cellular contractile events requires that vertebrate smooth and non-muscle myosin II can achieve an "off" state. To examine the role of the myosin rod in this process, we determined the minimal size at which a myosin molecule is capable of regulation via light chain phosphorylation. Expressed smooth muscle myosin subfragments with as many as 100 amino acids of the coiled-coil rod sequence did not dimerize and were active independently of phosphorylation. To test whether dimerization per se restores regulation of ATPase activity, mutants were expressed with varying lengths of rod sequence, followed by C-terminal leucine zippers to stabilize the coiled-coil. Dimerization restored partial regulation, but the presence of a length of rod approximately equal to the myosin head was necessary to achieve a completely off state. Partially regulated short dimers could be converted into fully regulated molecules by addition of native rod sequence after the zipper. These results suggest that the myosin rod mediates specific interactions with the head that are required to obtain the completely inactive state of vertebrate smooth and non-muscle myosins. If these interactions are prohibited under cellular conditions, unphosphorylated crossbridges can slowly cycle.
Figures


References
-
- Harrington W F, Rodgers M E, Davis J S. In: Molecular Mechanisms in Muscular Contraction. Squire J M, editor. New York: Macmillan; 1990. pp. 241–263.
-
- Itakura S, Yamakawa H, Toyoshima Y Y, Ishijima A, Kojima T, Harada Y, Yanagida T, Wakabayashi T, Sutoh S. Biochem Biophys Res Commun. 1993;196:1504–1510. - PubMed
-
- Trybus K M. J Muscle Res Cell Motil. 1994;15:587–594. - PubMed
-
- Cremo C R, Sellers J R, Facemyer K C. J Biol Chem. 1995;270:2171–2175. - PubMed
-
- O’Shea E K, Klemm J D, Kim P S, Alber T. Science. 1991;254:539–544. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

