Folding of the N-terminal, ligand-binding region of integrin alpha-subunits into a beta-propeller domain
- PMID: 8990162
- PMCID: PMC19237
- DOI: 10.1073/pnas.94.1.65
Folding of the N-terminal, ligand-binding region of integrin alpha-subunits into a beta-propeller domain
Abstract
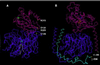
The N-terminal approximately 440 aa of integrin alpha subunits contain seven sequence repeats. These are predicted here to fold into a beta-propeller domain. A homologous domain from the enzyme phosphatidylinositol phospholipase D is predicted to have the same fold. The domains contain seven four-stranded beta-sheets arranged in a torus around a pseudosymmetry axis. The trimeric G-protein beta subunit (G beta) appears to be the most closely related beta-propeller. Integrin ligands and a putative Mg2+ ion are predicted to bind to the upper face of the beta-propeller. This face binds substrates in beta-propeller enzymes and is used by the G protein beta subunit to bind the G protein alpha subunit. The integrin alpha subunit I domain, which is structurally homologous to the G protein alpha subunit, is tethered to the top of the beta-propeller domain by a hinge that may allow movement of the domains relative to one another. The Ca2+-binding motifs in integrin alpha subunits are on the lower face of the beta-propeller.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

