The motor domain and the regulatory domain of myosin solely dictate enzymatic activity and phosphorylation-dependent regulation, respectively
- PMID: 8990166
- PMCID: PMC19241
- DOI: 10.1073/pnas.94.1.91
The motor domain and the regulatory domain of myosin solely dictate enzymatic activity and phosphorylation-dependent regulation, respectively
Abstract
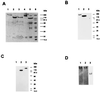
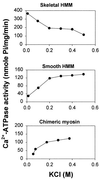
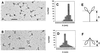
While the structures of skeletal and smooth muscle myosins are homologous, they differ functionally from each other in several respects, i.e., motor activities and regulation. To investigate the molecular basis for these differences, we have produced a skeletal/smooth chimeric myosin molecule and analyzed the motor activities and regulation of this myosin. The produced chimeric myosin is composed of the globular motor domain of skeletal muscle myosin (Met1-Gly773) and the C-terminal long alpha-helix domain of myosin subfragment 1 as well as myosin subfragment 2 (Gly773-Ser1104) and light chains of smooth muscle myosin. Both the actin-activated ATPase activity and the actin-translocating activity of the chimeric myosin were completely regulated by light chain phosphorylation. On the other hand, the maximum actin-activated ATPase activity of the chimeric myosin was the same as skeletal myosin and thus much higher than smooth myosin. These results show that the C-terminal light chain-associated domain of myosin head solely confers regulation by light chain phosphorylation, whereas the motor domain determines the rate of ATP hydrolysis. This is the first report, to our knowledge, that directly determines the function of the two structurally separated domains in myosin head.
Figures

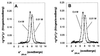
References
-
- Mooseker M S, Cheney R E. Annu Rev Cell Dev Biol. 1995;11:633–675. - PubMed
-
- Zot A S, Potter J D. Annu Rev Biophys Biophys Chem. 1987;16:535–559. - PubMed
-
- Hartshorne D J. In: Physiology of the Gastrointestinal Tract. 2nd Ed. Johnson L R, editor. Vol. 1. New York: Raven; 1987. pp. 423–482.
-
- Sellers J R, Adelstein R A. In: The Enzymes. Boyer P, Krebs E G, editors. Vol. 18. San Diego: Academic; 1987. pp. 381–418.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

