SC35-mediated reconstitution of splicing in U2AF-depleted nuclear extract
- PMID: 8990173
- PMCID: PMC19256
- DOI: 10.1073/pnas.94.1.133
SC35-mediated reconstitution of splicing in U2AF-depleted nuclear extract
Abstract
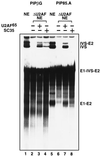
Assembly of the mammalian spliceosome is known to proceed in an ordered fashion through several discrete complexes, but the mechanism of this assembly process may not be universal. In an early step, pre-mRNAs are committed to the splicing pathway through association with U1 small nuclear ribonucleoprotein (snRNP) and non-snRNP splicing factors, including U2AF and members of the SR protein family. As a means of studying the steps of spliceosome assembly, we have prepared HeLa nuclear extracts specifically depleted of the splicing factor U2AF. Surprisingly, the SR protein SC35 can functionally substitute for U2AF65 in the reconstitution of pre-mRNA splicing in U2AF-depleted extracts. This reconstitution is substrate-specific and is reminiscent of the SC35-mediated reconstitution of splicing in extracts depleted of U1 snRNP. However, SC35 reconstitution of splicing in U2AF-depleted extracts is dependent on the presence of functional U1 snRNP. These observations suggest that there are at least three distinguishable mechanisms for the binding of U2 snRNP to the pre-mRNA, including U2AF-dependent and -independent pathways.
Figures



Similar articles
-
Characterization of a U2AF-independent commitment complex (E') in the mammalian spliceosome assembly pathway.Mol Cell Biol. 2005 Jan;25(1):233-40. doi: 10.1128/MCB.25.1.233-240.2005. Mol Cell Biol. 2005. PMID: 15601845 Free PMC article.
-
Multiple factors in the early splicing complex are involved in the nuclear retention of pre-mRNAs in mammalian cells.Genes Cells. 2011 Oct;16(10):1035-49. doi: 10.1111/j.1365-2443.2011.01548.x. Epub 2011 Sep 19. Genes Cells. 2011. PMID: 21929696
-
A U6 snRNA:pre-mRNA interaction can be rate-limiting for U1-independent splicing.Genes Dev. 1995 Sep 15;9(18):2314-23. doi: 10.1101/gad.9.18.2314. Genes Dev. 1995. PMID: 7557384
-
Mechanisms of fidelity in pre-mRNA splicing.Curr Opin Cell Biol. 2000 Jun;12(3):340-5. doi: 10.1016/s0955-0674(00)00097-1. Curr Opin Cell Biol. 2000. PMID: 10801464 Review.
-
A conserved mRNA export machinery coupled to pre-mRNA splicing.Cell. 2002 Feb 22;108(4):523-31. doi: 10.1016/s0092-8674(02)00627-x. Cell. 2002. PMID: 11909523 Review.
Cited by
-
SAM homeostasis is regulated by CFIm-mediated splicing of MAT2A.Elife. 2021 May 5;10:e64930. doi: 10.7554/eLife.64930. Elife. 2021. PMID: 33949310 Free PMC article.
-
Differential 3' splice site recognition of SMN1 and SMN2 transcripts by U2AF and U2 snRNP.RNA. 2009 Apr;15(4):515-23. doi: 10.1261/rna.1273209. Epub 2009 Feb 25. RNA. 2009. PMID: 19244360 Free PMC article.
-
G triplets located throughout a class of small vertebrate introns enforce intron borders and regulate splice site selection.Mol Cell Biol. 1997 Aug;17(8):4562-71. doi: 10.1128/MCB.17.8.4562. Mol Cell Biol. 1997. PMID: 9234714 Free PMC article.
-
A conditional role of U2AF in splicing of introns with unconventional polypyrimidine tracts.Mol Cell Biol. 2007 Oct;27(20):7334-44. doi: 10.1128/MCB.00627-07. Epub 2007 Aug 20. Mol Cell Biol. 2007. PMID: 17709389 Free PMC article.
-
Spliceosome assembly and regulation: insights from analysis of highly reduced spliceosomes.RNA. 2023 May;29(5):531-550. doi: 10.1261/rna.079273.122. Epub 2023 Feb 3. RNA. 2023. PMID: 36737103 Free PMC article. Review.
References
-
- Konarska M M, Sharp P A. Cell. 1986;46:845–855. - PubMed
-
- Guthrie C. Science. 1991;253:157–163. - PubMed
-
- Moore M J, Query C C, Sharp P A. In: The RNA World. Gesteland R F, Atkins J F, editors. Plainview, NY: Cold Spring Harbor Lab. Press; 1993. pp. 303–357.
-
- Krämer A. In: Pre-mRNA Processing. Lamond A I, editor. Austin, TX: Landes; 1995. pp. 35–64.
-
- Ruby S R, Abelson J. Science. 1988;242:1028–1035. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

