Resistance of actin to cleavage during apoptosis
- PMID: 8990178
- PMCID: PMC19266
- DOI: 10.1073/pnas.94.1.157
Resistance of actin to cleavage during apoptosis
Abstract
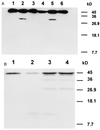
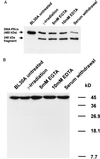
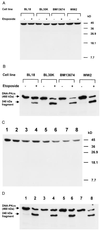
A small number of cellular proteins present in the nucleus, cytosol, and membrane fraction are specifically cleaved by the interleukin-1beta-converting enzyme (ICE)-like family of proteases during apoptosis. Previous results have demonstrated that one of these, the cytoskeletal protein actin, is degraded in rat PC12 pheochromocytoma cells upon serum withdrawal. Extracts from etoposide-treated U937 cells are also capable of cleaving actin. It was assumed that cleavage of actin represented a general phenomenon, and a mechanism coordinating proteolytic, endonucleolytic, and morphological aspects of apoptosis was proposed. We demonstrate here that actin is resistant to degradation in several different human cells induced to undergo apoptosis in response to a variety of stimuli, including Fas ligation, serum withdrawal, cytotoxic T-cell killing, and DNA damage. On the other hand, cell-free extracts from these cells and the ICE-like protease CPP32 were capable of cleaving actin in vitro. We conclude that while actin contains cleavage sites for ICE-like proteases, it is not degraded in vivo in human cells either because of lack of access of these proteases to actin or due to the presence of other factors that prevent degradation.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

