Transgenic studies with a keratin promoter-driven growth hormone transgene: prospects for gene therapy
- PMID: 8990189
- PMCID: PMC19291
- DOI: 10.1073/pnas.94.1.219
Transgenic studies with a keratin promoter-driven growth hormone transgene: prospects for gene therapy
Abstract
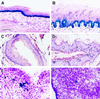
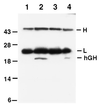
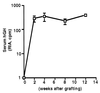
Keratinocytes are potentially appealing vehicles for the delivery of secreted gene products because they can be transferred to human skin by the relatively simple procedure of grafting. Adult human keratinocytes can be efficiently propagated in culture with sufficient proliferative capacity to produce enough epidermis to cover the body surface of an average adult. However, the feasibility of delivering secreted proteins through skin grafting rests upon (i) the strength of the promoter in keratinocytes and (ii) the efficiency of protein transport through the basement membrane of the stratified epithelium and into the bloodstream. In this paper, we use transgenic technology to demonstrate that the activity of the human keratin 14 promoter remains high in adult skin and that keratinocyte-derived human growth hormone (hGH) can be produced, secreted, and transported to the bloodstream of mice with efficiency that is sufficient to exceed by an order of magnitude the circulating hGH concentration in growing children. Transgenic skin grafts from these adults continue to produce and secrete hGH stably, at approximately 1/10 physiological levels in the bloodstream of nontransgenic recipient mice. These studies underscore the utility of the keratin 14 promoter for expressing foreign transgenes in keratinocytes and demonstrate that keratinocytes can be used as effective vehicles for transporting factors to the bloodstream and for eliciting metabolic changes. These findings have important implications for considering the keratinocyte as a possible vehicle for gene therapy.
Figures


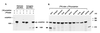




References
-
- Rochat A, Kobayashi K, Barrandon Y. Cell. 1994;76:1063–1073. - PubMed
-
- Rheinwald J G, Green H. Cell. 1975;6:331–343. - PubMed
-
- Rheinwald J G, Green H. Nature (London) 1977;265:421–424. - PubMed
-
- Gallico G G, O’Connor N E, Compton C C, Kehinde O, Green H. N Eng J Med. 1984;311:448–451. - PubMed
-
- Morgan J R, Barrandon Y, Green H, Mulligan R C. Science. 1987;237:1476–1479. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

