Surface dynamics in living acinar cells imaged by atomic force microscopy: identification of plasma membrane structures involved in exocytosis
- PMID: 8990206
- PMCID: PMC19328
- DOI: 10.1073/pnas.94.1.316
Surface dynamics in living acinar cells imaged by atomic force microscopy: identification of plasma membrane structures involved in exocytosis
Abstract
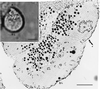
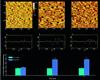
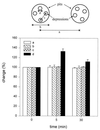
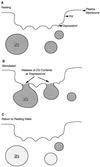
The dynamics at the plasma membrane resulting from secretory vesicle docking and fusion and compensatory endocytosis has been difficult to observe in living cells primarily due to limited resolution at the light microscopic level. Using the atomic force microscope, we have been able to image and record changes in plasma membrane structure at ultrahigh resolution after stimulation of secretion from isolated pancreatic acinar cells. "Pits" measuring 500-2000 nm and containing 3-20 depressions measuring 100-180 nm in diameter were observed only at the apical region of acinar cells. The time course of an increase and decrease in "depression" size correlated with an increase and decrease of amylase secretion from live acinar cells. Depression dynamics and amylase release were found to be regulated in part by actin. No structural changes were identified at the basolateral region of these cells. Our results suggest depressions to be the fusion pores identified earlier in mast cells by freeze-fracture electron microscopy and by electrophysiological measurements. The atomic force microscope has enabled us to observe plasma membrane dynamics of the exocytic process in living cells in real time.
Figures

Comment in
-
Cellular and molecular mechanics by atomic force microscopy: capturing the exocytotic fusion pore in vivo?Proc Natl Acad Sci U S A. 1997 Jan 7;94(1):9-10. doi: 10.1073/pnas.94.1.9. Proc Natl Acad Sci U S A. 1997. PMID: 8990151 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

