Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation
- PMID: 8994059
- PMCID: PMC6573165
- DOI: 10.1523/JNEUROSCI.17-03-01046.1997
Amyloid beta-peptide impairs glucose transport in hippocampal and cortical neurons: involvement of membrane lipid peroxidation
Abstract
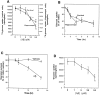
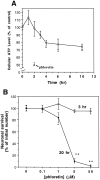
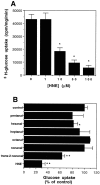
A deficit in glucose uptake and a deposition of amyloid beta-peptide (A beta) each occur in vulnerable brain regions in Alzheimer's disease (AD). It is not known whether mechanistic links exist between A beta deposition and impaired glucose transport. We now report that A beta impairs glucose transport in cultured rat hippocampal and cortical neurons by a mechanism involving membrane lipid peroxidation. A beta impaired 3H-deoxy-glucose transport in a concentration-dependent manner and with a time course preceding neurodegeneration. The decrease in glucose transport was followed by a decrease in cellular ATP levels. Impairment of glucose transport, ATP depletion, and cell death were each prevented in cultures pretreated with antioxidants. Exposure to FeSO4, an established inducer of lipid peroxidation, also impaired glucose transport. Immunoprecipitation and Western blot analyses showed that exposure of cultures to A beta induced conjugation of 4-hydroxynonenal (HNE), an aldehydic product of lipid peroxidation, to the neuronal glucose transport protein GLUT3. HNE induced a concentration-dependent impairment of glucose transport and subsequent ATP depletion. Impaired glucose transport was not caused by a decreased energy demand in the neurons, because ouabain, which inhibits Na+/K(+)-ATPase activity and thereby reduces neuronal ATP hydrolysis rate, had little or no effect on glucose transport. Collectively, the data demonstrate that lipid peroxidation mediates A beta-induced impairment of glucose transport in neurons and suggest that this action of A beta may contribute to decreased glucose uptake and neuronal degeneration in AD.
Figures



Similar articles
-
Impairment of glucose and glutamate transport and induction of mitochondrial oxidative stress and dysfunction in synaptosomes by amyloid beta-peptide: role of the lipid peroxidation product 4-hydroxynonenal.J Neurochem. 1997 Jul;69(1):273-84. doi: 10.1046/j.1471-4159.1997.69010273.x. J Neurochem. 1997. PMID: 9202320
-
4-hydroxynonenal, a lipid peroxidation product, impairs glutamate transport in cortical astrocytes.Glia. 1998 Feb;22(2):149-60. doi: 10.1002/(sici)1098-1136(199802)22:2<149::aid-glia6>3.0.co;2-2. Glia. 1998. PMID: 9537835
-
4-Hydroxynonenal, an aldehydic product of membrane lipid peroxidation, impairs glutamate transport and mitochondrial function in synaptosomes.Neuroscience. 1997 Oct;80(3):685-96. doi: 10.1016/s0306-4522(97)00065-1. Neuroscience. 1997. PMID: 9276486
-
Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death.Neurobiol Aging. 2002 Sep-Oct;23(5):655-64. doi: 10.1016/s0197-4580(01)00340-2. Neurobiol Aging. 2002. PMID: 12392766 Review.
-
Amyloid beta peptide, 4-hydroxynonenal and apoptosis.Curr Alzheimer Res. 2006 Sep;3(4):359-64. doi: 10.2174/156720506778249506. Curr Alzheimer Res. 2006. PMID: 17017866 Review.
Cited by
-
Oxidative stress and lipid peroxidation are upstream of amyloid pathology.Neurobiol Dis. 2015 Dec;84:109-19. doi: 10.1016/j.nbd.2015.06.013. Epub 2015 Jun 21. Neurobiol Dis. 2015. PMID: 26102023 Free PMC article.
-
DNA polymerase β deficiency leads to neurodegeneration and exacerbates Alzheimer disease phenotypes.Nucleic Acids Res. 2015 Jan;43(2):943-59. doi: 10.1093/nar/gku1356. Epub 2014 Dec 30. Nucleic Acids Res. 2015. PMID: 25552414 Free PMC article.
-
Glucose deprivation induces mitochondrial dysfunction and oxidative stress in PC12 cell line.J Cell Mol Med. 2003 Jan-Mar;7(1):49-56. doi: 10.1111/j.1582-4934.2003.tb00202.x. J Cell Mol Med. 2003. PMID: 12767261 Free PMC article.
-
Oxidative stress in rat cortical neurons and astrocytes induced by paraquat in vitro.Neurotox Res. 2002 Feb;4(1):1-13. doi: 10.1080/10298420290007574. Neurotox Res. 2002. PMID: 12826488
-
SIRT3 Haploinsufficiency Aggravates Loss of GABAergic Interneurons and Neuronal Network Hyperexcitability in an Alzheimer's Disease Model.J Neurosci. 2020 Jan 15;40(3):694-709. doi: 10.1523/JNEUROSCI.1446-19.2019. Epub 2019 Dec 9. J Neurosci. 2020. PMID: 31818974 Free PMC article.
References
-
- Barger SW, Mattson MP. Induction of neuroprotective κB-dependent transcription by secreted forms of the Alzheimer’s β-amyloid precursor. Mol Brain Res. 1996;40:116–126. - PubMed
-
- Behl C, Davis J, Lesley R, Schubert D. Hydrogen peroxide mediates amyloid β protein toxicity. Cell. 1994;77:817–827. - PubMed
-
- Benzi G, Moretti A. Age- and peroxidative stress-related modifications of the cerebral enzymatic activities linked to mitochondria and the glutathione system. Free Radic Biol Med. 1995;19:77–101. - PubMed
-
- Blass JP. Metabolic alterations common to neural and non-neural cells in Alzheimer’s disease. Hippocampus. 1993;3:45–54. - PubMed
-
- Bowling AC, Beal MF. Bioenergetic and oxidative stress in neurodegenerative diseases. Life Sci. 1995;56:1151–1171. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
