Costimulation of T cell activation by integrin-associated protein (CD47) is an adhesion-dependent, CD28-independent signaling pathway
- PMID: 8996237
- PMCID: PMC2211576
- DOI: 10.1084/jem.185.1.1
Costimulation of T cell activation by integrin-associated protein (CD47) is an adhesion-dependent, CD28-independent signaling pathway
Abstract
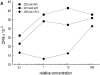
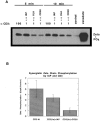
The integrin-associated protein (IAP, CD47) is a 50-kD plasma membrane protein with a single extracellular immunoglobulin variable (IgV)-like domain, a multiply membrane-spanning segment, and alternatively spliced short cytoplasmic tails. On neutrophils, IAP has been shown to function in a signaling complex with beta 3 integrins. However, the function of IAP on T cells, which express little or no beta 3 integrin, is not yet defined. Here, we show that mAbs recognizing IAP can enhance proliferation of primary human T cells in the presence of low levels of anti-CD3, but have no effect on T cell proliferation on their own. Together with suboptimal concentrations of anti-CD3, engagement of IAP also enhances IL-2 production in Jurkat cells, an apparently integrin-independent function of IAP. Nonetheless, costimulation by IAP ligation requires cell adhesion. IAP costimulation does not require CD28. Furthermore, anti-IAP, but not anti-CD28, synergizes with suboptimal anti-CD3 to enhance tyrosine phosphorylation of the CD3 zeta chain and the T cell-specific tyrosine kinase Zap70. Ligation of human IAP transfected into the hemoglobin-specific 3.L2 murine T cell hybridoma costimulates activation for IL-2 secretion both with anti-CD3 and with antigenic peptides on antigen-presenting cells (APCs). Moreover, ligation of IAP but not CD28 can convert antagonist peptides into agonists in 3.L2 cells. Using costimulation by IAP ligation as an assay to analyze the structure-function relationships in IAP signaling, we find that both the extracellular and multiply membrane-spanning domains of IAP are necessary for synergy with the antigen receptor, but the alternatively spliced cytoplasmic tails are not. These data demonstrate that IAP ligation initiates an adhesion-dependent costimulatory pathway distinct from CD28. We hypothesize that anti-IAP generates the costimulatory signal because it modulates interactions of the IgV domain with other plasma membrane molecules; this in turn activates effector functions of the multiply membrane-spanning domain of IAP. This model may have general significance for how IAP functions in cell activation.
Figures










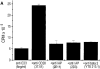
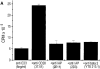


References
-
- Lindberg FP, Lublin DM, Telen MJ, Veile RA, Miller YE, Donis-Keller H, Brown EJ. Rh-related antigen CD47 is the signal-transducer Integrin-associated protein. J Biol Chem. 1994;269:1567–1570. - PubMed
-
- Schwartz MA, Brown EJ, Fazeli B. A 50 kDa integrin-associated protein is required for integrin-regulated calcium entry in endothelial cells. J Biol Chem. 1993;268:19931–19934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

