Scleroderma autoantigens are uniquely fragmented by metal-catalyzed oxidation reactions: implications for pathogenesis
- PMID: 8996243
- PMCID: PMC2196102
- DOI: 10.1084/jem.185.1.71
Scleroderma autoantigens are uniquely fragmented by metal-catalyzed oxidation reactions: implications for pathogenesis
Abstract
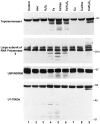
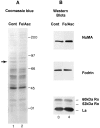
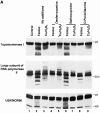
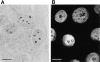
The observation that revelation of immunocryptic epitopes in self antigens may initiate the autoimmune response has prompted the search for processes which induce novel fragmentation of autoantigens as potential initiators of autoimmunity. The reversible ischemia reperfusion which characterizes scleroderma has focused attention on reactive oxygen species as molecules which might induce autoantigen fragmentation. We demonstrate that several of the autoantigens targeted in diffuse scleroderma are uniquely susceptible to cleavage by reactive oxygen species, in a metal-dependent manner. Multiple features of the fragmentation reaction and its inhibition indicate that these autoantigens possess metal-binding sites, which focus metal-catalyzed oxidation reactions (and consequent fragmentation) to specific regions of the antigens. These data suggest that the autoantibody response in scleroderma is the immune marker of unique protein fragmentation, induced by ischemia reperfusion in the presence of appropriate metals, and focus attention on abnormal metal status as a potential pathogenic principle in this disease.
Figures




References
-
- Radic MZ, Weigert M. Genetic and structural evidence for antigen selection of anti-DNA antibodies. Annu Rev Immunol. 1994;12:487–520. - PubMed
-
- Diamond B, Katz JB, Paul E, Aranow C, Lustgarten D, Scharff MD. The role of somatic mutation in the pathogenic anti-DNA response. Annu Rev Immunol. 1992;10:731–757. - PubMed
-
- Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729–766. - PubMed
-
- Gammon G, Sercarz EE. How some T cells escape tolerance induction. Nature (Lond) 1989;342:183–185. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

