Loss of ATP diphosphohydrolase activity with endothelial cell activation
- PMID: 8996251
- PMCID: PMC2196106
- DOI: 10.1084/jem.185.1.153
Loss of ATP diphosphohydrolase activity with endothelial cell activation
Abstract
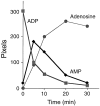
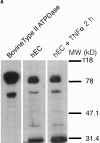
Quiescent endothelial cells (EC) regulate blood flow and prevent intravascular thrombosis. This latter effect is mediated in a number of ways, including expression by EC of thrombomodulin and heparan sulfate, both of which are lost from the EC surface as part of the activation response to proinflammatory cytokines. Loss of these anticoagulant molecules potentiates the procoagulant properties of the injured vasculature. An additional thromboregulatory factor, ATP diphosphohydrolase (ATPDase; designated as EC 3.6.1.5) is also expressed by quiescent EC, and has the capacity to degrade the extracellular inflammatory mediators ATP and ADP to AMP, thereby inhibiting platelet activation and modulating vascular thrombosis. We describe here that the antithrombotic effects of the ATPDase, like heparan sulfate and thrombomodulin, are lost after EC activation, both in vitro and in vivo. Because platelet activation and aggregation are important components of the hemostatic changes that accompany inflammatory diseases, we suggest that the loss of vascular ATPDase may be crucial for the progression of vascular injury.
Figures








References
-
- Preissner KT. Anticoagulant potential of endothelial cell membrane components. Haemostasis. 1988;18:271–306. - PubMed
-
- Ihrcke NS, Wrenshall LE, Lindman BJ, Platt JL. Role of heparan sulfate in immune system-blood vessel interactions. Immunol Today. 1993;14:500–505. - PubMed
-
- Esmon CT. Protein-S and protein-C–biochemistry, physiology, and clinical manifestation of deficiencies. Trends Cardiovasc Med. 1992;2:214–219. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

