Subunit stoichiometry of human muscle chloride channels
- PMID: 8997668
- PMCID: PMC2217051
- DOI: 10.1085/jgp.109.1.93
Subunit stoichiometry of human muscle chloride channels
Abstract
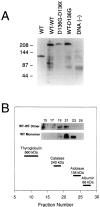
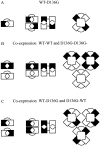
Voltage-gated Cl- channels belonging to the ClC family appear to function as homomultimers, but the number of subunits needed to form a functional channel is controversial. To determine subunit stoichiometry, we constructed dimeric human skeletal muscle Cl- channels in which one subunit was tagged by a mutation (D136G) that causes profound changes in voltage-dependent gating. Sucrose-density gradient centrifugation experiments indicate that both monomeric and dimeric hClC-1 channels in their native configurations exhibit similar sedimentation properties consistent with a multimeric complex having a molecular mass of a dimer. Expression of the heterodimeric channel in a mammalian cell line results in a homogenous population of Cl- channels exhibiting novel gating properties that are best explained by the formation of heteromultimeric channels with an even number of subunits. Heteromultimeric channels were not evident in cells cotransfected with homodimeric WT-WT and D136G-D136G constructs excluding the possibility that functional hClC-1 channels are assembled from more than two subunits. These results demonstrate that the functional hClC-1 unit consists of two subunits.
Figures







References
-
- Adachi S, Uchida S, Ito H, Hata M, Hiroe M, Marumo F, Sasaki S. Two isoforms of a chloride channel predominantly expressed in thick ascending limb of Henle's loop and collecting ducts of rat kidney. J Biol Chem. 1994;269:17677–17683. - PubMed
-
- Chahine M, George AL, Jr, Zhou M, Ji S, Sun W, Barchi RL, Horn R. Sodium channel mutations in paramyotonia congenita uncouple inactivation from activation. Neuron. 1994;12:281–294. - PubMed

