Cloning, characterization, and expression of a G-protein-coupled receptor from Lymnaea stagnalis and identification of a leucokinin-like peptide, PSFHSWSamide, as its endogenous ligand
- PMID: 9006965
- PMCID: PMC6793731
- DOI: 10.1523/JNEUROSCI.17-04-01197.1997
Cloning, characterization, and expression of a G-protein-coupled receptor from Lymnaea stagnalis and identification of a leucokinin-like peptide, PSFHSWSamide, as its endogenous ligand
Abstract
Neuropeptides are known to be important signaling molecules in several neural systems of the pond snail Lymnaea stagnalis. Although the functions of these peptides have been studied in many neurons, the nature of the postsynaptic signal transduction is mainly unknown. The cloning and characterization of neuropeptide receptors in Lymnaea thus would be very valuable in further elucidating peptidergic pathways. Indirect evidence suggests that these neuropeptides operate via G-protein-coupled mechanisms indicating the presence of G-protein-coupled receptors as the initial postsynaptic targets. Here we describe the cloning of a neuropeptide receptor from Lymnaea and the isolation of an endogenous ligand. This peptide, PSFHSWSamide, belongs to the leucokinin family of peptides, and, thus, this Lymnaea receptor is the first example of a leucokinin-like neuropeptide receptor, representing a new subfamily of G-protein-coupled neuropeptide receptors.
Figures


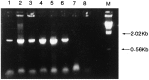

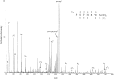
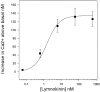
References
-
- Benjamin PR, Burke JF. Alternative mRNA splicing of the FMRFamide gene and its role in neuropeptidergic signalling in a defined network. BioEssays. 1994;16:335–342. - PubMed
-
- Blackburn MB, Wagner RM, Shasanowitz J, Kochansky JP, Hunt DF, Raina AK. The isolation and identification of 3 diuretic kinins from the abdominal ventral nerve cord of adult Helicoverpa zea. J Insect Physiol. 1996;41:723–730.
-
- Blitz DM, Christie AE, Marder EM, Nusbaum MP. Distribution and effects of tachykinin-like peptides in the stomatogastric nervous system of the crab, Cancer borealis. J Comp Neurol. 1995;354:282–294. - PubMed
-
- Chen Y, Veenstra JA, Davis NT, Hagedorn HH. A comparative study of leucokinin-immunoreactive neurons in insects. Cell Tissue Res. 1994;276:69–83. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
