Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain
- PMID: 9006968
- PMCID: PMC6793739
- DOI: 10.1523/JNEUROSCI.17-04-01226.1997
Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain
Abstract
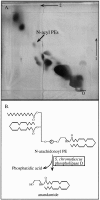
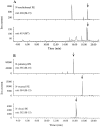
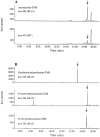
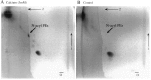
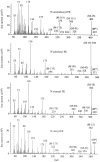
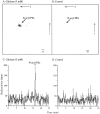
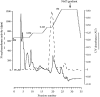
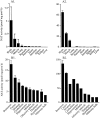
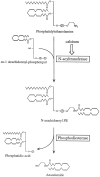
It has been suggested that anandamide (N-arachidonoylethanolamine), an endogenous cannabinoid substance, may be produced through Ca2+-stimulated hydrolysis of the phosphatidylethanolamine (PE) derivative N-arachidonoyl PE. The presence of N-arachidonoyl PE in adult brain tissue and the enzyme pathways that underlie its biosynthesis are, however, still undetermined. We report here that rat brain tissue contains both anandamide (11 +/- 7 pmol/gm wet tissue) and N-arachidonoyl PE (22 +/- 16 pmol/gm), as assessed by gas chromatography/mass spectrometry. We describe a N-acyltransferase activity in brain that catalyzes the biosynthesis of N-arachidonoyl PE by transferring an arachidonate group from the sn-1 carbon of phospholipids to the amino group of PE. We also show that sn-1 arachidonoyl phospholipids are present in brain, where they constitute approximately 0.5% of total phospholipids. N-acyltransferase activity is Ca2+ dependent and is enriched in brain and testis. Within the brain, N-acyltransferase activity is highest in brainstem; intermediate in cortex, striatum, hippocampus, medulla, and cerebellum; and lowest in thalamus, hypothalamus, and olfactory bulb. Pharmacological inhibition of N-acyltransferase activity in primary cultures of cortical neurons prevents Ca2+-stimulated N-arachidonoyl PE biosynthesis. Our results demonstrate, therefore, that rat brain tissue contains the complement of enzymatic activity and lipid substrates necessary for the biosynthesis of the anandamide precursor N-arachidonoyl PE. They also suggest that biosynthesis of N-arachidonoyl PE and formation of anandamide are tightly coupled processes, which may concomitantly be stimulated by elevations in intracellular Ca2+ occurring during neural activity.
Figures



References
-
- Aveldano de Caldironi MI, Bazan NG. α-Methyl-p-tyrosine inhibits the production of free arachidonic acid and diacylglycerols in brain after a single electroconvulsive shock. Neurochem Res. 1979;4:213–221. - PubMed
-
- Blank ML, Cress EA, Robinson M, Snyder F. Metabolism of unique diarachidonoyl and linoleoylarachidonoyl species of ethanolamine and choline phosphoglycerides in rat testes. Biochim Biophys Acta. 1985;833:366–371. - PubMed
-
- Cadas H, Schinelli S, Piomelli D. Membrane localization of N-acylphosphatidylethanolamine in central neurons: studies with exogenous phospholipases. J Lipid Med Cell Signal. 1996b;14:63–70. - PubMed
-
- Chilton FH, Murphy RC. Stimulated production and natural occurrence of 1,2-diarachidonoylglycerophosphorylcholine in human neutrophils. Biochem Biophys Res Commun. 1987;145:1126–1133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
