G1/S cell cycle blockers and inhibitors of cyclin-dependent kinases suppress camptothecin-induced neuronal apoptosis
- PMID: 9006970
- PMCID: PMC6793728
- DOI: 10.1523/JNEUROSCI.17-04-01256.1997
G1/S cell cycle blockers and inhibitors of cyclin-dependent kinases suppress camptothecin-induced neuronal apoptosis
Abstract
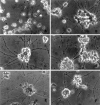
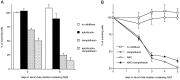
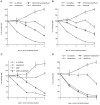
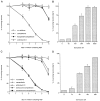
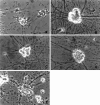
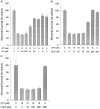
Previous studies have demonstrated that G1/S cell cycle blockers and inhibitors of cyclin-dependent kinases (CDKs) prevent the death of nerve growth factor (NGF)-deprived PC12 cells and sympathetic neurons, suggesting that proteins normally involved in the cell cycle may also serve to regulate neuronal apoptosis. Past findings additionally demonstrate that DNA-damaging agents, such as the DNA topoisomerase (topo-I) inhibitor camptothecin, also induce neuronal apoptosis. In the present study, we show that camptothecin-induced apoptosis of PC12 cells, sympathetic neurons, and cerebral cortical neurons is suppressed by the G1/S blockers deferoxamine and mimosine, as well as by the CDK-inhibitors flavopiridol and olomoucine. In each case, the IC50 values were similar to those reported for inhibition of death induced by NGF-deprivation. In contrast, other agents that arrest DNA synthesis, such as aphidicolin and N-acetylcysteine, failed to block death. This suggests that the inhibition of DNA synthesis per se is insufficient to provide protection from camptothecin. We find additionally that the cysteine aspartase family protease inhibitor zVAD-fmk inhibits apoptosis evoked by NGF-deprivation but not camptothecin treatment. Thus, despite their shared sensitivity to G1/S blockers and CDK inhibitors, the apoptotic pathways triggered by these two causes of death diverge at the level of the cysteine aspartase. In summary, neuronal apoptosis induced by the DNA-damaging agent camptothecin appears to involve signaling pathways that normally control the cell cycle. The consequent death signals of such deregulation, however, are different from those that result from trophic factor deprivation.
Figures





Similar articles
-
Multiple pathways of neuronal death induced by DNA-damaging agents, NGF deprivation, and oxidative stress.J Neurosci. 1998 Feb 1;18(3):830-40. doi: 10.1523/JNEUROSCI.18-03-00830.1998. J Neurosci. 1998. PMID: 9437005 Free PMC article.
-
Inhibitors of cyclin-dependent kinases promote survival of post-mitotic neuronally differentiated PC12 cells and sympathetic neurons.J Biol Chem. 1996 Apr 5;271(14):8161-9. doi: 10.1074/jbc.271.14.8161. J Biol Chem. 1996. PMID: 8626506
-
Cell cycle blockers mimosine, ciclopirox, and deferoxamine prevent the death of PC12 cells and postmitotic sympathetic neurons after removal of trophic support.J Neurosci. 1996 Feb 1;16(3):1150-62. doi: 10.1523/JNEUROSCI.16-03-01150.1996. J Neurosci. 1996. PMID: 8558244 Free PMC article.
-
CDK inhibitors: cell cycle arrest versus apoptosis.Cell Cycle. 2002 Mar-Apr;1(2):122-3. doi: 10.4161/cc.1.2.115. Cell Cycle. 2002. PMID: 12429920 Review. No abstract available.
-
Preclinical and clinical development of the cyclin-dependent kinase inhibitor flavopiridol.Clin Cancer Res. 2004 Jun 15;10(12 Pt 2):4270s-4275s. doi: 10.1158/1078-0432.CCR-040020. Clin Cancer Res. 2004. PMID: 15217973 Review.
Cited by
-
Activation of serine/threonine protein phosphatase-1 is required for ceramide-induced survival of sympathetic neurons.Biochem J. 2005 Feb 1;385(Pt 3):685-93. doi: 10.1042/BJ20040929. Biochem J. 2005. PMID: 15361069 Free PMC article.
-
Cell cycle reactivation in mature neurons: a link with brain plasticity, neuronal injury and neurodegenerative diseases?Neurosci Bull. 2011 Jun;27(3):185-96. doi: 10.1007/s12264-011-1002-z. Neurosci Bull. 2011. PMID: 21614101 Free PMC article. Review.
-
Regulation of the latency-reactivation cycle by products encoded by the bovine herpesvirus 1 (BHV-1) latency-related gene.J Neurovirol. 2011 Dec;17(6):535-45. doi: 10.1007/s13365-011-0060-3. Epub 2011 Dec 3. J Neurovirol. 2011. PMID: 22139602 Review.
-
Baseline cell proliferation rates and response to UV differ in lymphoblastoid cell lines derived from healthy individuals of extreme constitution types.Cell Cycle. 2021 May;20(9):903-913. doi: 10.1080/15384101.2021.1909884. Epub 2021 Apr 18. Cell Cycle. 2021. PMID: 33870855 Free PMC article.
-
ATM and the epigenetics of the neuronal genome.Mech Ageing Dev. 2013 Oct;134(10):434-9. doi: 10.1016/j.mad.2013.05.005. Epub 2013 May 23. Mech Ageing Dev. 2013. PMID: 23707635 Free PMC article. Review.
References
-
- Aller P, Rius C, Mata F, Zorrilla A, Cabanas C, Bellon T, Bernabeu C. Camptothecin induces differentiation and stimulates the expression of differentiation-related genes in U-937 human promonocytic leukemia cells. Cancer Res. 1992;52:1245–1251. - PubMed
-
- Brooks SF, Gibson LA, Rubin LL. Apoptosis induced by NGF-withdrawal from differentiated PC12 cells involves activation of P34cdc2 kinase. Soc Neurosci Abstr. 1993;1:885.
-
- Chen X, Bargonetti J, Prives C. P53, through 21 (WAF/CIP1), induces cyclin D1 synthesis. Cancer Res. 1995;55:4257–4263. - PubMed
-
- Cheng B, Mattson MP. NGF and bFGF protect rat hippocampal and human cortical neurons against hypoglycemic damage by stabilizing calcium homeostasis. Neuron. 1991;7:1031–1041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
